Nomenclature of Alcohols
Nomenclature of alcohols is pretty straightforward. We take an alkane and add the “-ol” ending. This way, we get methanol from methane and ethanol from ethane.
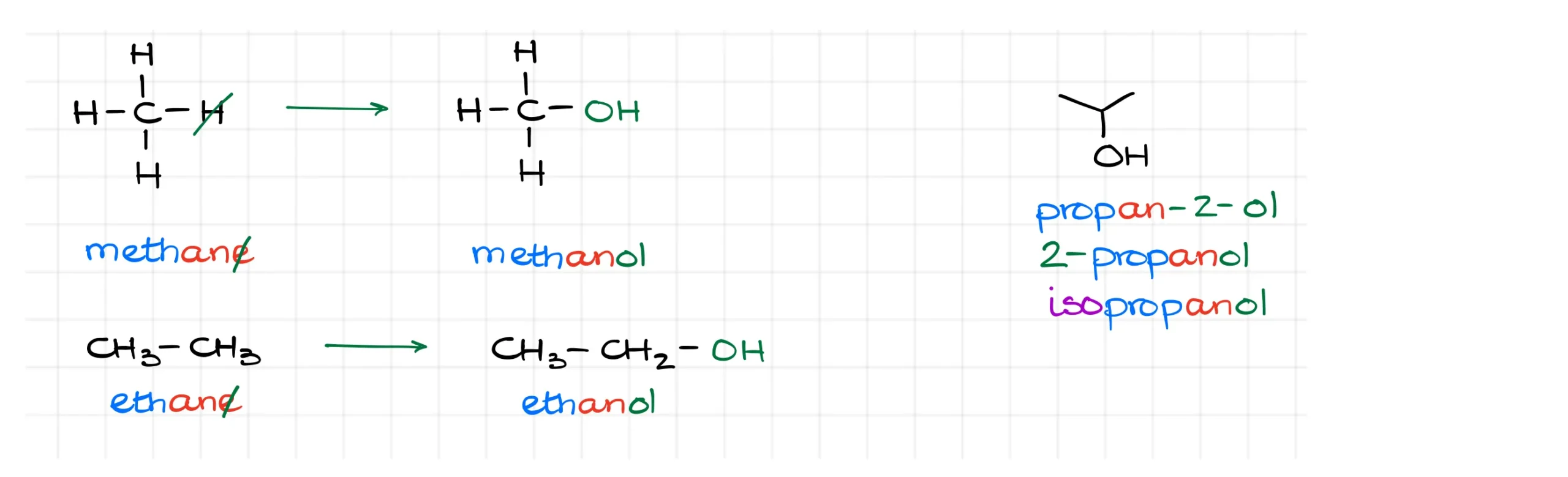
But once a molecule has more atoms, we also need to indicate the position of the –OH group. For instance, here we have propan-2-ol, also known as 2-propanol or isopropanol.
Since alcohols have been known for thousands of years, and the naming of organic substances evolved much like natural language, you’ll often see common names alongside systematic ones. In this tutorial, I’ll focus on the systematic IUPAC names.
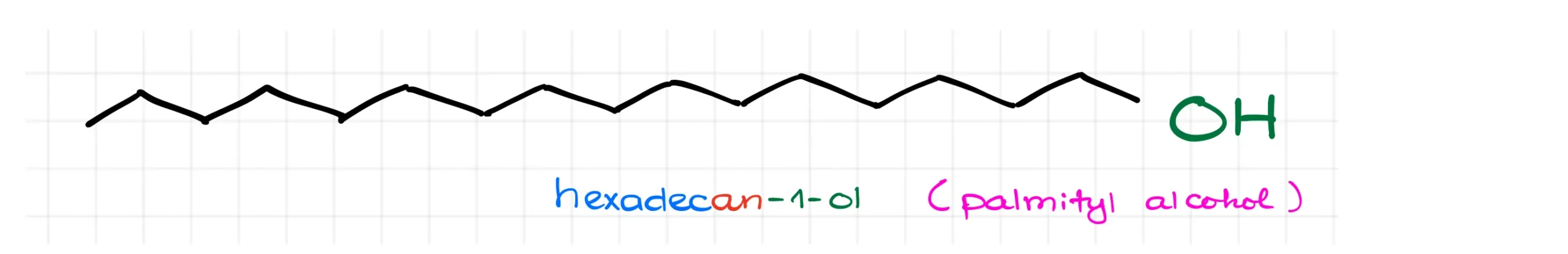
Here’s another example: hexadecan-1-ol, also known as palmityl alcohol. It’s used in cosmetics as an opacifier, or a substance that makes a product more opaque. Titanium dioxide was traditionally used for this purpose, but because of its potential toxicity and carcinogenic effects, industries are now exploring alternatives.
Let’s go over a few examples to see how it all works in more complex cases.
Example 1
Let’s look at this first example. Since by now you should already be comfortable with the basics of organic nomenclature, I won’t explain every step in detail, but instead focus on the rules specific to alcohols.
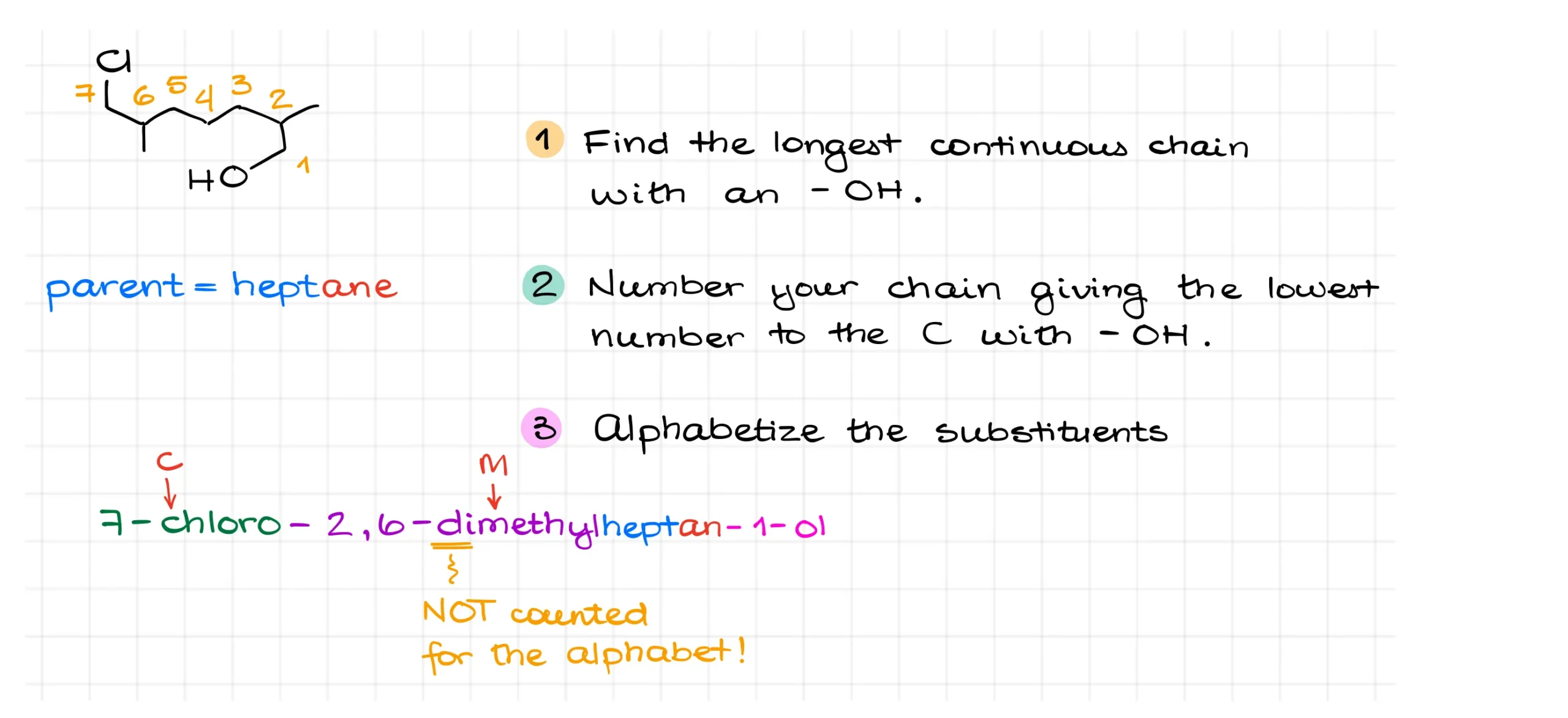
Step one is to find the longest continuous chain containing the –OH group. That means we must include the carbon that holds the alcohol when choosing our parent chain. Here, that gives us a heptane backbone.
Next, we number the chain so that the carbon with the –OH group has the lowest possible number. This makes the parent chain heptan-1-ol.
Now let’s add substituents. In this molecule, there are methyl groups at carbons 2 and 6, and a chlorine at carbon 7. Remember that when alphabetizing substituents, numeric prefixes like “di” don’t count. So “chloro” is alphabetized under “C” and “methyl” under “M.”
Putting everything together, we get 7-chloro-2,6-dimethylheptan-1-ol.
Example 2
Now let’s try another example. Here we have a molecule with two –OH groups. Again, we find the longest chain containing both groups. Numbering the chain from either side gives the same locants for the –OH groups, so we look at substituents as the tiebreaker.
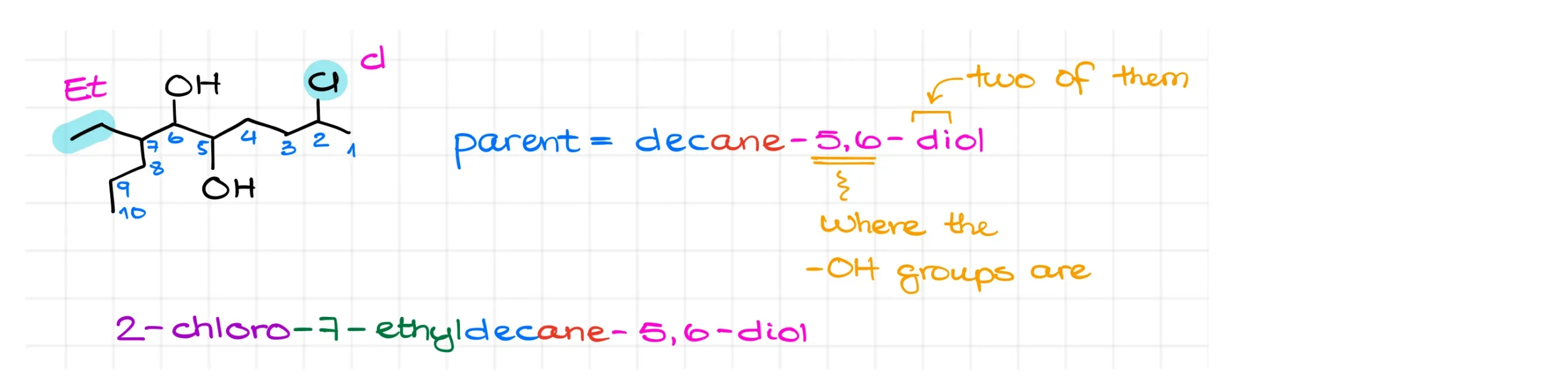
Numbering from the left places substituents at carbons 2 and 7, while numbering from the right places them at 4 and 9. Since 2 and 7 are lower, we use that system.
This gives us a parent name of decane-5,6-diol. Notice that I specified both positions of the –OH groups, 5 and 6, and used the prefix “di” to show there are two of them.
For substituents, there is an ethyl group at carbon 7 and a chlorine at carbon 2. Alphabetizing gives the final name: 2-chloro-7-ethyldecane-5,6-diol.
Cyclic Alcohol Example
Now let’s look at a cyclic example. If a cycle has only one –OH group, we don’t need to specify its position, because numbering always starts from the carbon with the alcohol. Although, some instructors will require you to say “1” for the location of the alcohol. The IUPAC rules keep going back and forth on this, so different instructors sometimes have different opinions on the necessity of the “1” in cases like this one.
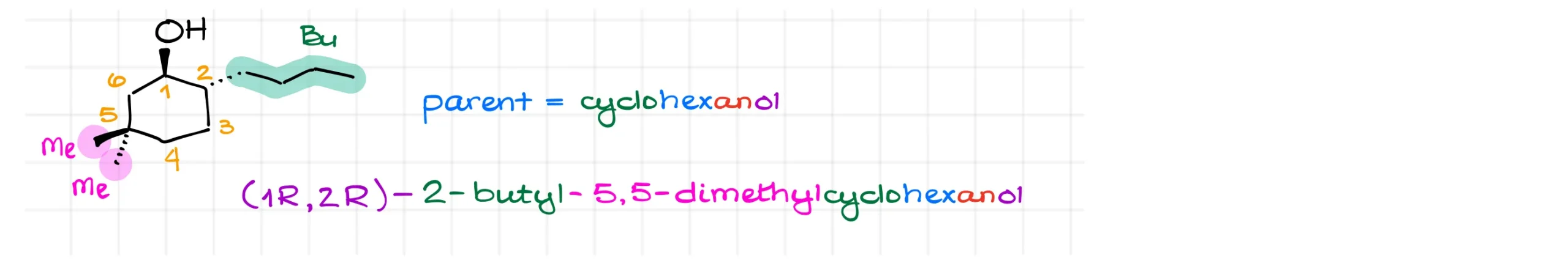
So, for a simple case, the parent is just cyclohexanol. We then number the ring toward the nearest substituent, which in this case is clockwise.
This gives us a butyl substituent at carbon 2 and two methyl groups at carbon 5. Alphabetizing these substituents gives the name 2-butyl-5,5-dimethylcyclohexanol.
If stereochemistry is specified, we must include it. For example, this molecule might be named (1R,2R)-2-butyl-5,5-dimethylcyclohexanol. Remember to assign stereodescriptors properly. If your instructor doesn’t say to ignore stereochemistry, they’ll expect it in your answer, and you’ll lose points if you leave it out.
Example with Multiple Functional Groups
So far, we’ve named open-chain alcohols and cyclic alcohols. Now let’s look at molecules with more than one type of functional group.
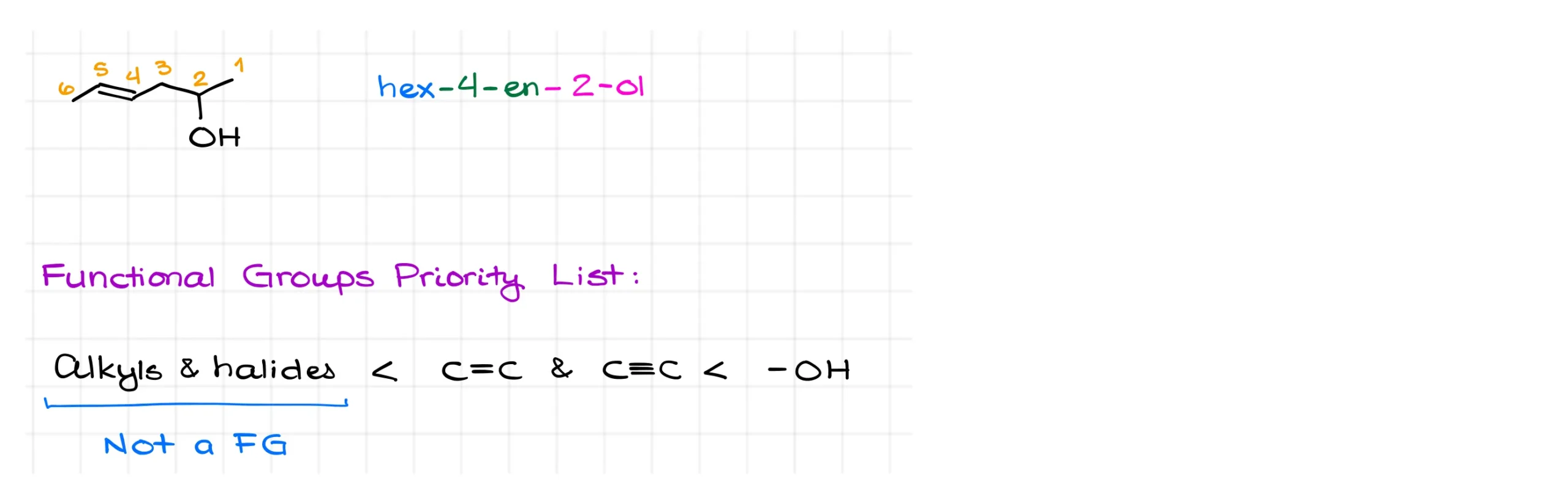
Here’s an example with both an alcohol and an alkene. Functional groups follow their own priority order in nomenclature, which is different from the CIP rules for stereochemistry.
Alkyl groups and halides are not considered functional groups in this context. Alkenes and alkynes come next, with alkenes given slightly higher priority only when numbering is otherwise the same. Alcohols, however, outrank both alkenes and alkynes.
That means when numbering a chain with both an alkene and an alcohol, the alcohol takes priority. In this case, we number from the right end to give the –OH group the lowest number. Since alkenes and alkynes don’t have prefix forms, we can end up with multiple suffixes in the name. The highest priority functional group always takes the final suffix position.
So here, the correct name is hex-4-en-2-ol.
Summary

To summarize:
Always prioritize alcohols over alkenes and alkynes when numbering.
Indicate the position of the –OH group before the “-ol” ending.
If there are multiple –OH groups, list them all with the appropriate numeric prefix.
And don’t forget stereochemistry when it’s required.
