Oxymercuration-Reduction of Alkenes
Even though the chances of seeing someone perform an oxymercuration reaction in a modern lab are lower than your chances of meeting a live dinosaur the next time you poke your nose outside, this reaction is a must-know for any organic chemistry student—and it will definitely be on your next exam. So in this tutorial, I’m going to tell you everything you need to know about this reaction, and probably a little bit more.
Mechanism of the Oxymercuration of Alkenes
Let’s start with a simplified mechanism.
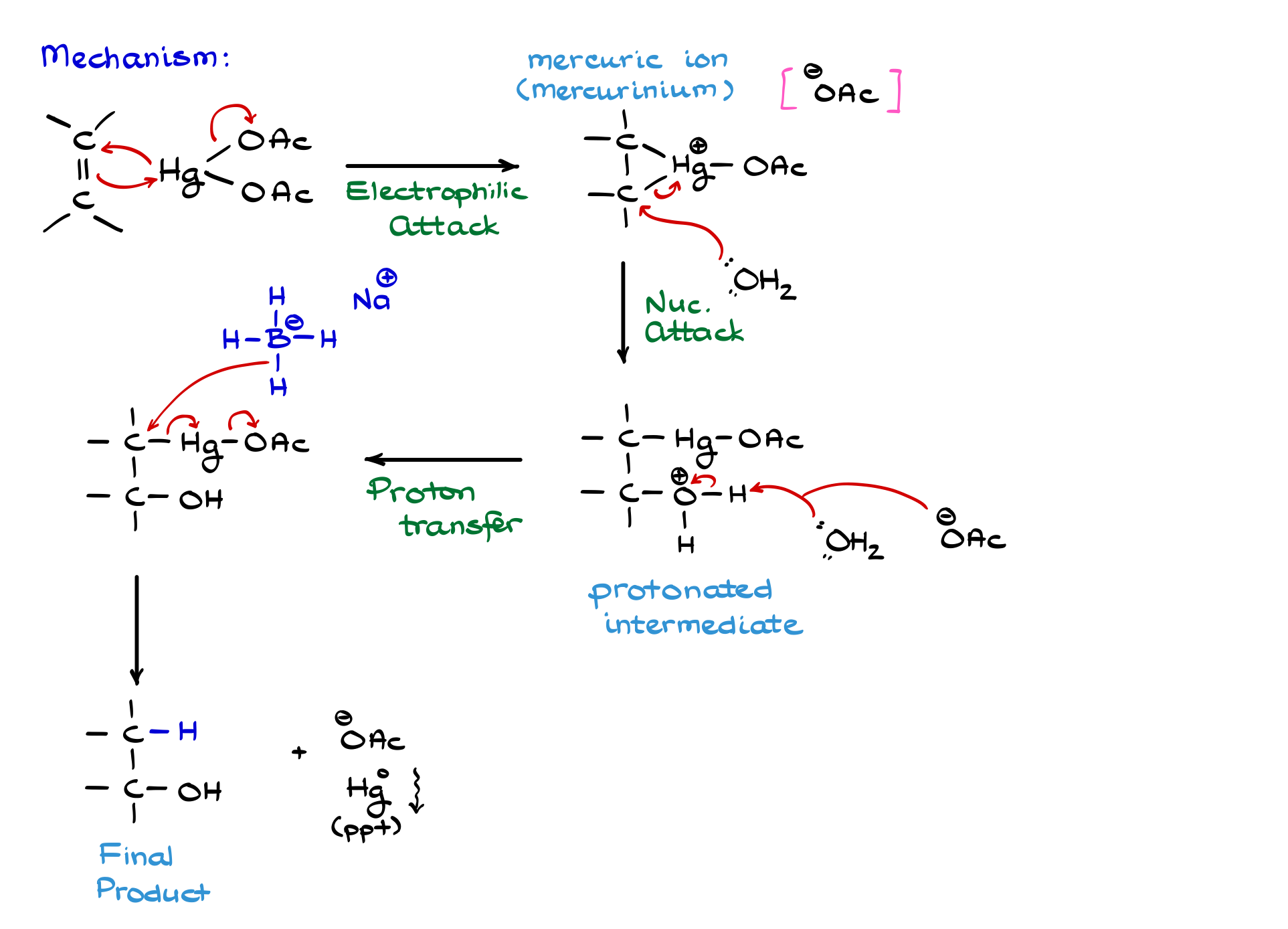
This reaction consists of two distinct phases. In the first phase, we react our alkene with mercury. Mechanistically, this step is very similar to the halogenation of an alkene. Here, the double bond acts as a nucleophile, donating electron density toward the mercury. One of the acetates drops off, and then mercury re-attacks one of the carbons of the double bond.
We’re not showing electron pairs on mercury because those are d-electrons. It’s a bit of a blasphemy to draw d-electrons, but trust me, they’re there. So, we’re going to show the reaction starting our arrow from mercury, not from the electron pair like we normally do.
As a result of this electrophilic attack on the double bond, we form a mercuric ion. It’s also called a mercurinium ion. Both terms are synonymous and perfectly fine to use. We also generate some free-floating acetate. Just like in halogenation or oxyhalogenation, the concentration of acetate is relatively low here. Since we’re performing this reaction in excess water (because it’s an aqueous solution) water acts as our primary nucleophile to open the mercuric ion.
When water attacks, it goes for the more substituted carbon, as you’d expect. The electrons move back onto the mercury, forming a new carbon–oxygen bond. And just like in an oxyhalogenation reaction, we end up with a protonated intermediate. We naturally deprotonate it with either another water molecule or an acetate ion—either works. Some textbooks prefer water, others use acetate. Same difference for our purposes.
The proton on our intermediate falls off, giving us the final product of the first step, which looks something like this. No one in their right mind would try to isolate this intermediate, though—organomercury compounds are incredibly toxic. So, we immediately work it up while it’s still in the reaction mixture.
The last step is the reduction, typically done with sodium borohydride (NaBH₄) in basic media. This step is sometimes called the demercuration reaction. So, this process is called either oxymercuration-reduction or oxymercuration-demercuration. Both mean the same thing.
Most instructors won’t ask you to draw the mechanism for that last step. But if they do, we usually teach a simplified version: the hydride ion from borohydride attacks a carbon, causing a cascade of electron shifts. As a result, we get an alcohol, a floating acetate ion, and metallic mercury that settles as little silvery droplets.
While elemental mercury isn’t as toxic as organomercury compounds, it’s definitely not a holiday gift either. There’s a whole complicated process for safely disposing of mercury, which is why people nowadays avoid this type of chemistry. Other, less toxic methods exist to achieve the same result, but in sophomore organic chemistry, we still teach this classical method, even if it feels a bit archaic.
Example of the Oxymercuration
Now, let’s look at an example.
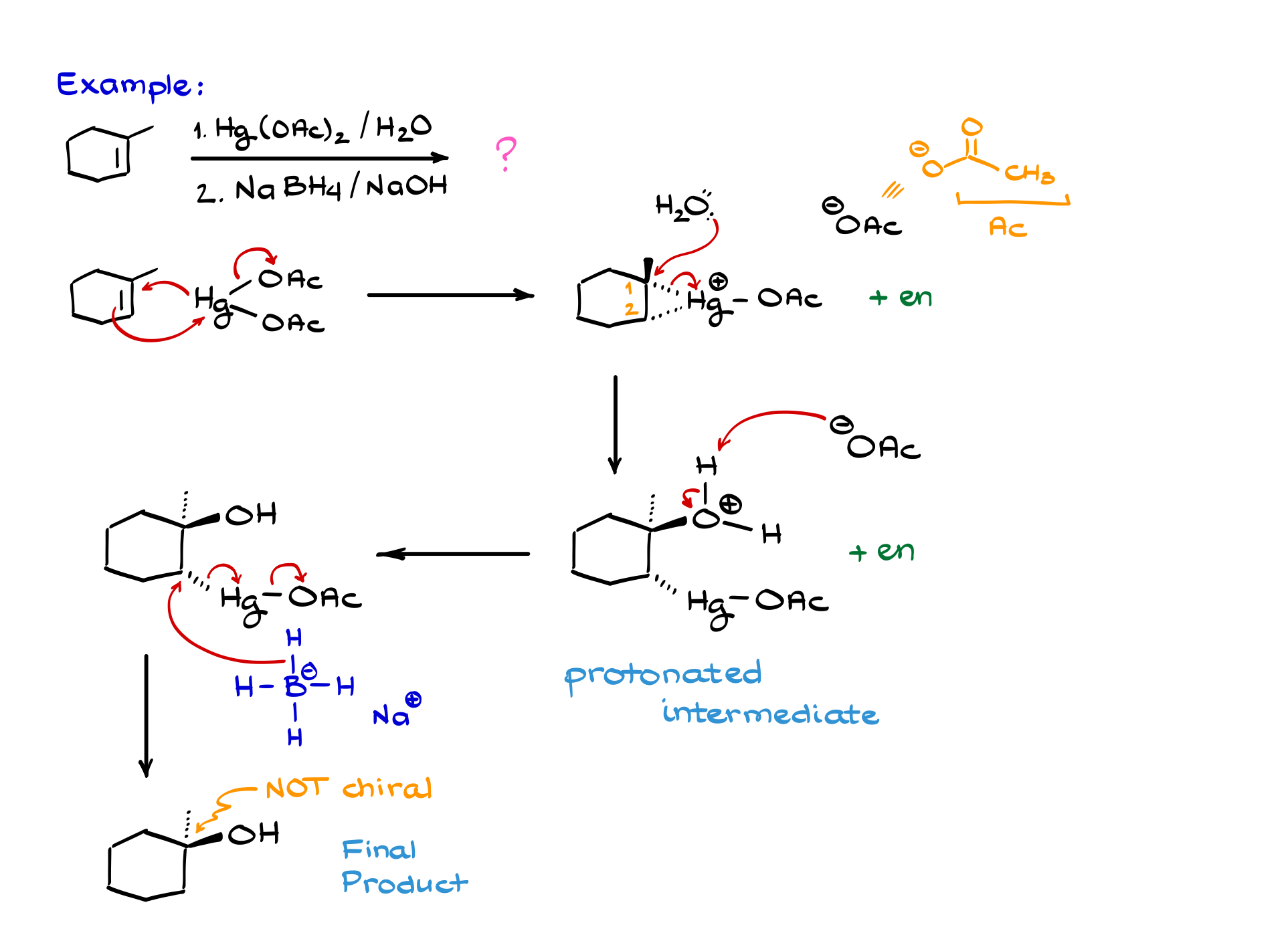
We’ll use my favorite, 1-methylcyclohexene, and mercury in this oxymercuration reaction. We start with the electrophilic attack by mercury on the alkene. Electrons from the alkene go to mercury, one of the acetates falls off, and mercury back-attacks a carbon, forming a three-membered ring.
This intermediate can form enantiomers because mercury can attack from the front or back face. In this example, mercury attacks from the back face, so the methyl group points away from us. Meanwhile, water and acetate ions float around in solution.
Next, water attacks the more substituted carbon of our three-membered ring. The electrons go back onto the mercury, opening the ring and giving us the protonated intermediate and its enantiomer.
Then, either water or acetate deprotonates the intermediate, forming a neutral molecule. Let’s say acetate does the job here. We get a molecule that looks like this.
Finally, we bring in sodium borohydride. The hydride attacks a carbon, electrons shift to mercury, and then onto the acetate. This is a shortcut version of the real mechanism, but it’s what we generally teach.
Our final product looks like this. If you automatically added “+ enantiomer,” you’d be wrong—this carbon isn’t chiral, so no enantiomers are formed. Always check your stereochemistry carefully; this is a common trap on exams. Instructors love to test this.
Now, you might be asking, “Why even bother with this reaction?” Mercury is toxic, and this process needs an extra step to remove it. Why not just use a simple hydration reaction?
Good question. The reason isn’t because organic chemists enjoy making things harder. It’s because, unlike simple hydration, this reaction doesn’t involve carbocation intermediates, so there’s no risk of rearrangement.
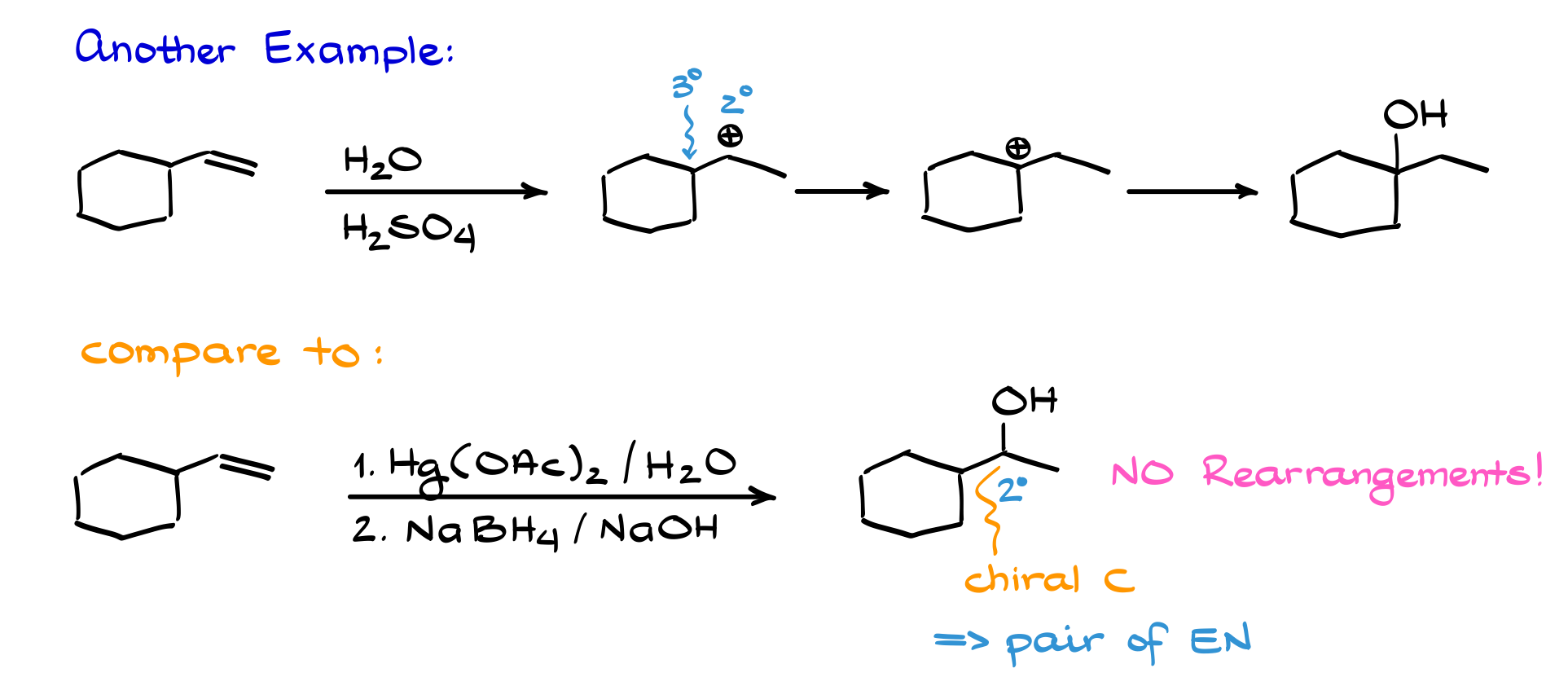
Let me show you. If we did a simple hydration on this molecule, we’d first form a secondary carbocation next to a tertiary position. That secondary carbocation would immediately undergo a hydride shift to become a more stable tertiary carbocation. Follow the mechanism, and you get a product that looks like this.
Now, compare that to doing the same reaction with oxymercuration. No carbocation forms, so no rearrangement occurs. We end up with OH on the secondary carbon. And here’s the kicker: that carbon is chiral, so the product is a racemic mixture of enantiomers.
One last point: this reaction overall is not stereospecific. The first step, forming the mercuric ion and opening it, is stereospecific—OH and mercury end up on opposite sides. But the second step, the reduction with NaBH₄, isn’t stereospecific. So the overall result is a non-stereospecific reaction.

If your product has multiple chiral centers, you’ll get all combinations. For example, if you start with this molecule and do oxymercuration-reduction, you’ll end up with four stereoisomers because you’ve made two chiral centers.
Practice Questions
Predict the major product in each of the following oxymercuration reactions paying extra attention to the stereochemistry of the products.
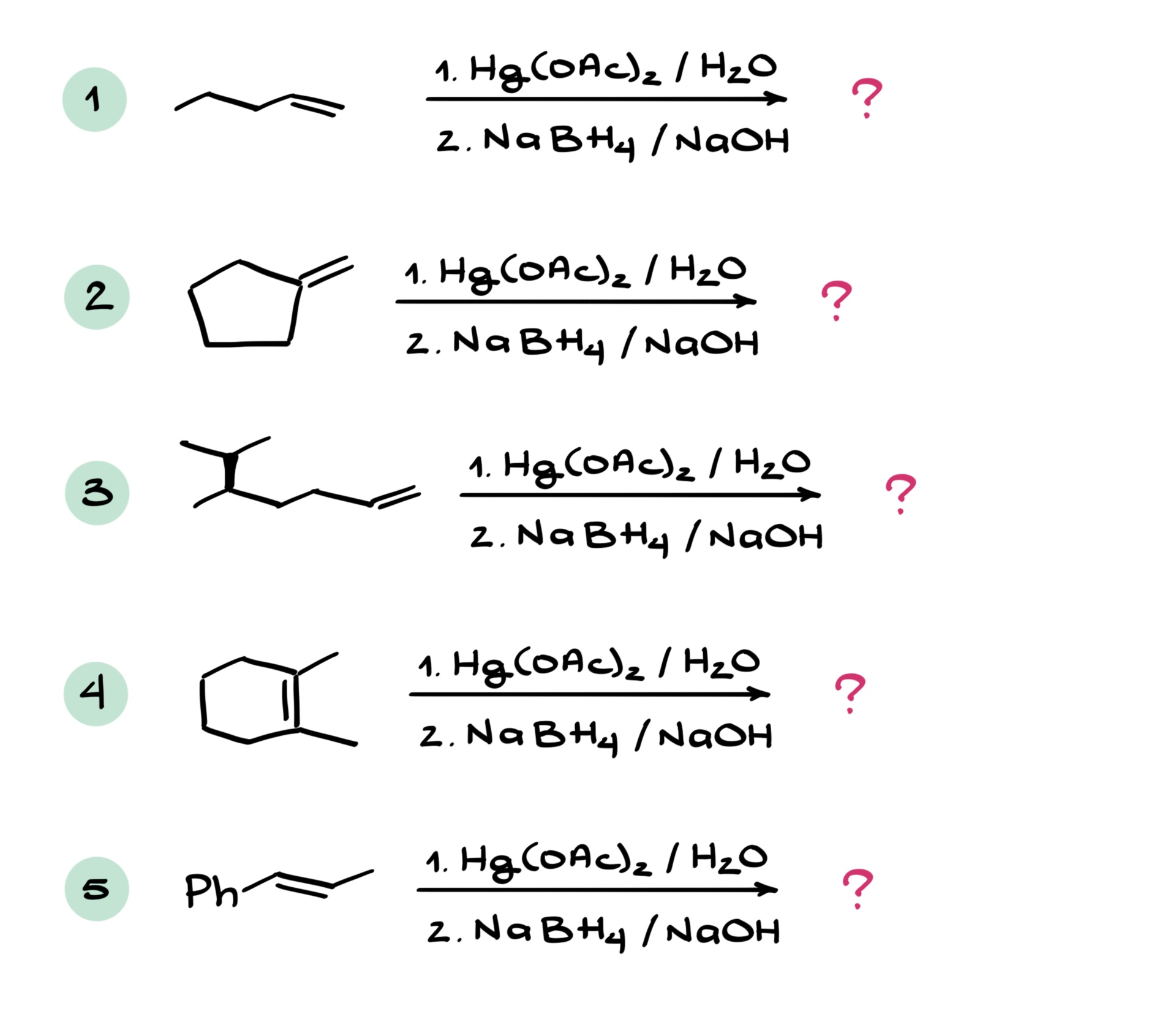
Would you like to see the answers and check your work?
Sign up or login if you’re already a member and unlock all members-only content!
