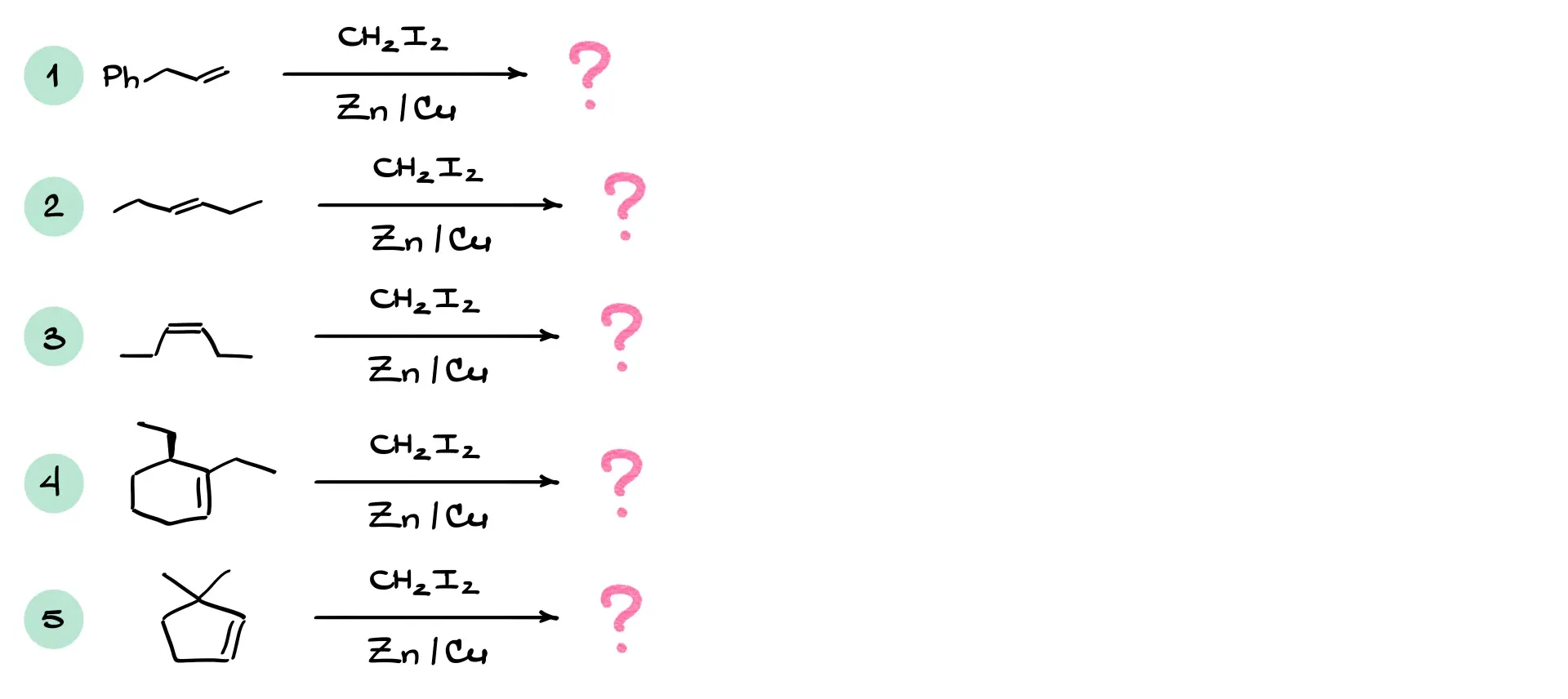The Simmons-Smith Reaction and Cyclopropanation of Alkenes
In this tutorial, we’re going to explore two closely related and fascinating reactions in organic chemistry: cyclopropanation and the Simmons–Smith reaction.
While these two processes aren’t exactly the same, they share the same goal—they both transform alkenes into cyclopropanes. Even though these reactions might seem a bit niche, they show up frequently on exams and are essential tools in any organic chemist’s repertoire. So let’s take a closer look and make sure you understand everything you need to ace these topics on your test.
The Simmons-Smith Reaction
We’ll begin with the Simmons–Smith reaction. This method relies on a mixture of diiodomethane and a zinc–copper alloy to generate a reactive carbenoid species. The zinc atom inserts itself between the carbon and iodine atoms of the diiodomethane, forming a new intermediate that acts similarly to a carbene. You don’t need to memorize the details of this transformation—just remember that it creates a reactive species ready to attack alkenes. This reaction can technically be carried out using pure zinc, but the success of that approach depends heavily on the condition of the zinc surface. Often, we use ultrasound to activate the reaction and improve its efficiency. Most typically, we’re going to see the zinc-copper alloy (Zn/Cu) that facilitates this reaction much better than pure zinc.
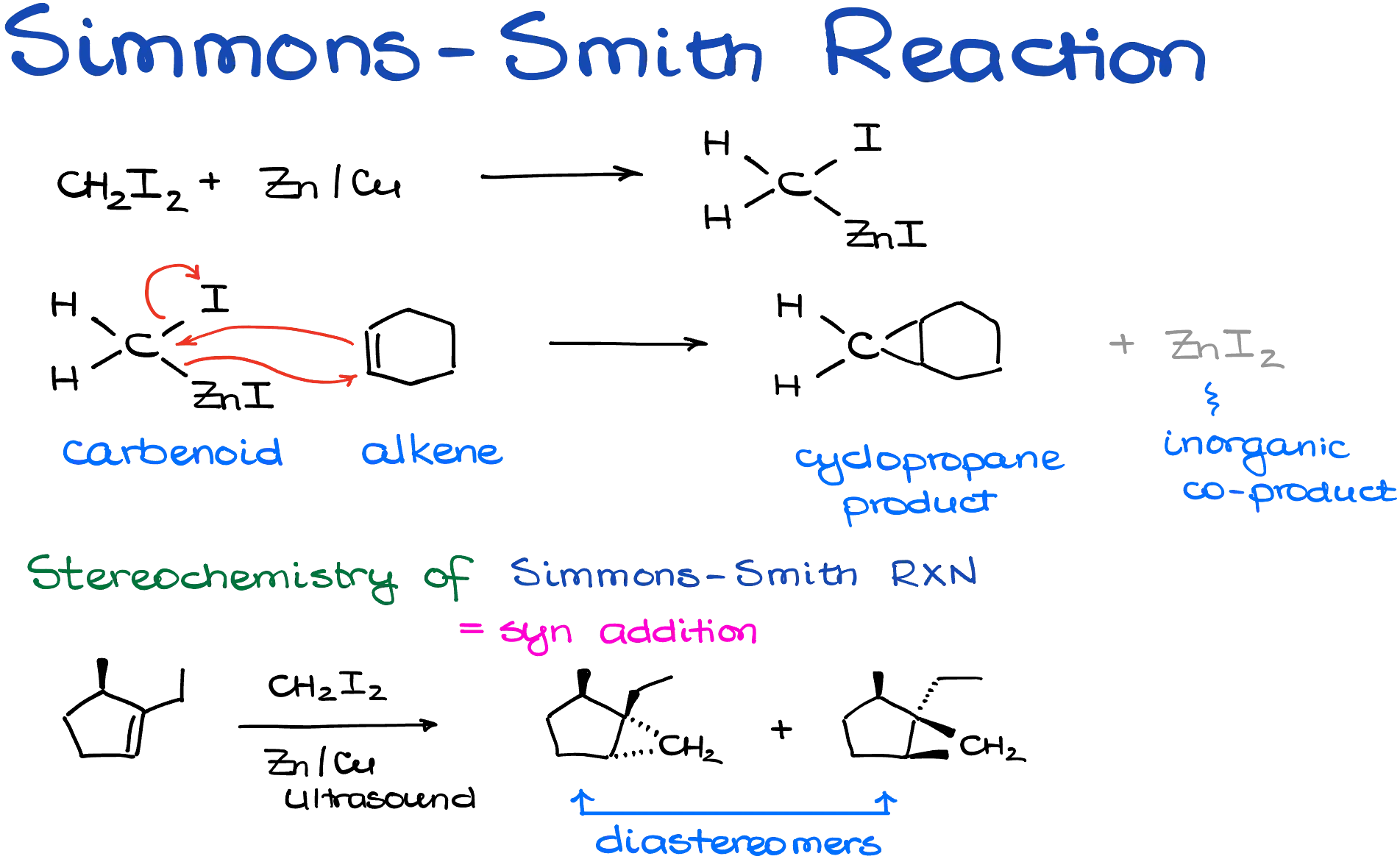
Once this zinc–carbenoid species is formed, it reacts with an alkene through a concerted mechanism. That means both new bonds—from the CH₂ unit to each carbon of the alkene—form at the same time. The result is a cyclopropane ring. Zinc iodide is produced as a byproduct, but it’s irrelevant to us and usually left out of reaction schemes.
Simmons-Smith Examples
From a stereochemical perspective, this reaction is stereospecific and classified as a syn addition. This makes sense when you think about the geometry: you can only insert the CH₂ unit from one face of the molecule to create a three-membered ring.
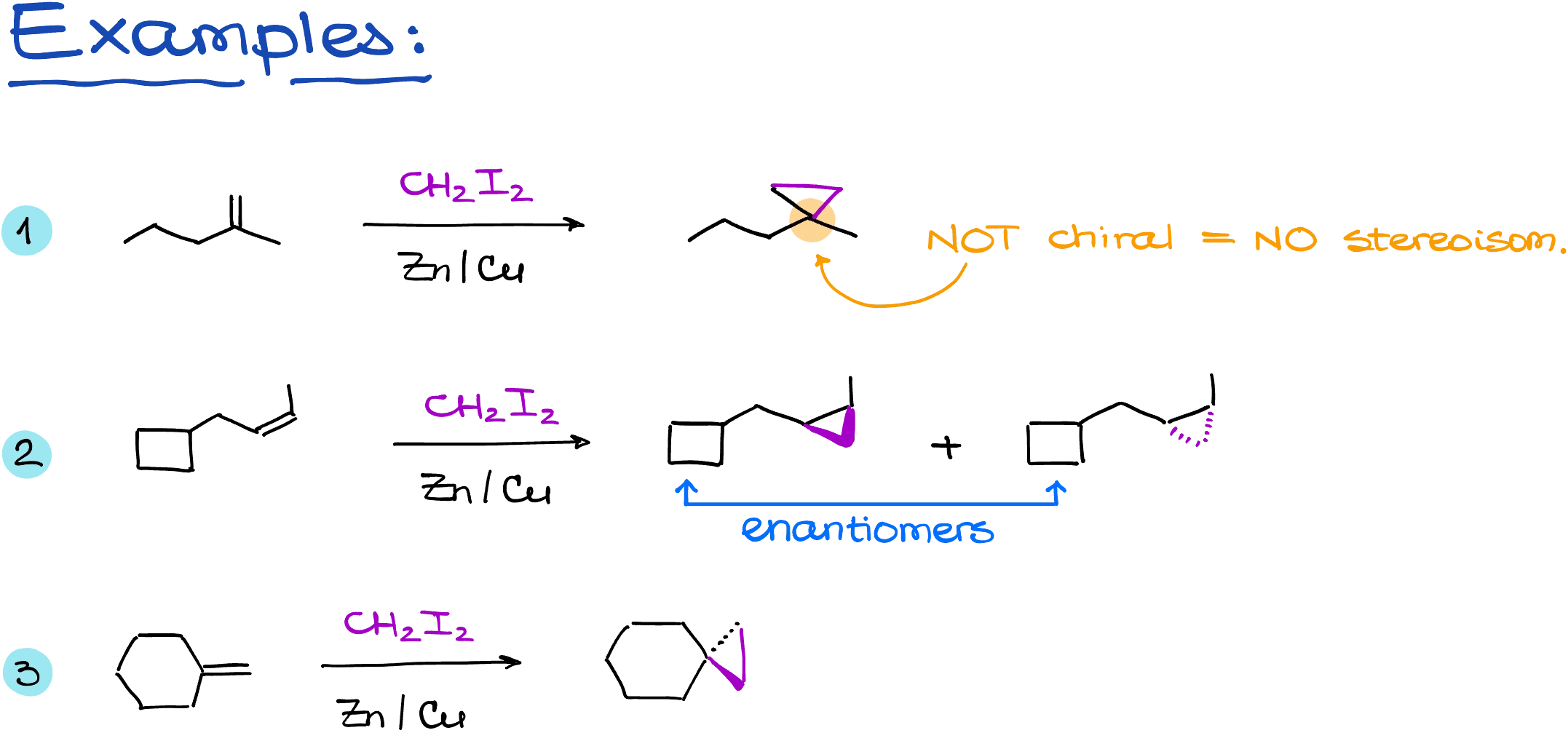
For example, when adding across a double bond, you can form two different stereoisomers depending on which face the CH₂ approaches from. These products are diastereomers. In other cases, such as when the product lacks chirality, you won’t get any stereoisomers. Some cyclopropane products will be meso compounds, having internal planes of symmetry. Others can be spirocyclic and non-chiral, depending on the structure of the starting alkene.
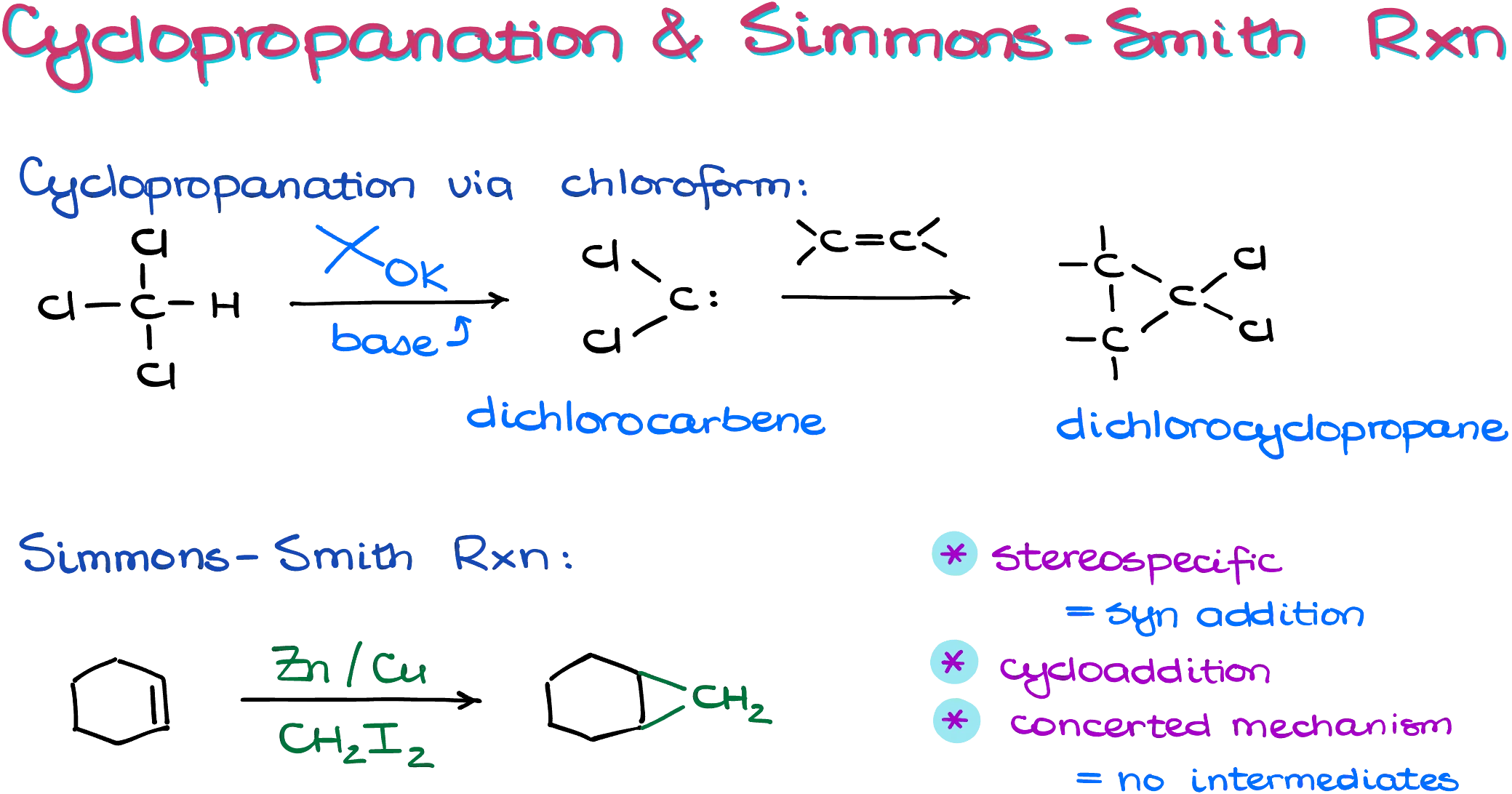
Cyclopropanation via Dichlorocarbene
Now let’s shift to the other major cyclopropanation method, which doesn’t have a fancy name. As the name suggests, this route involves generating a true carbene.
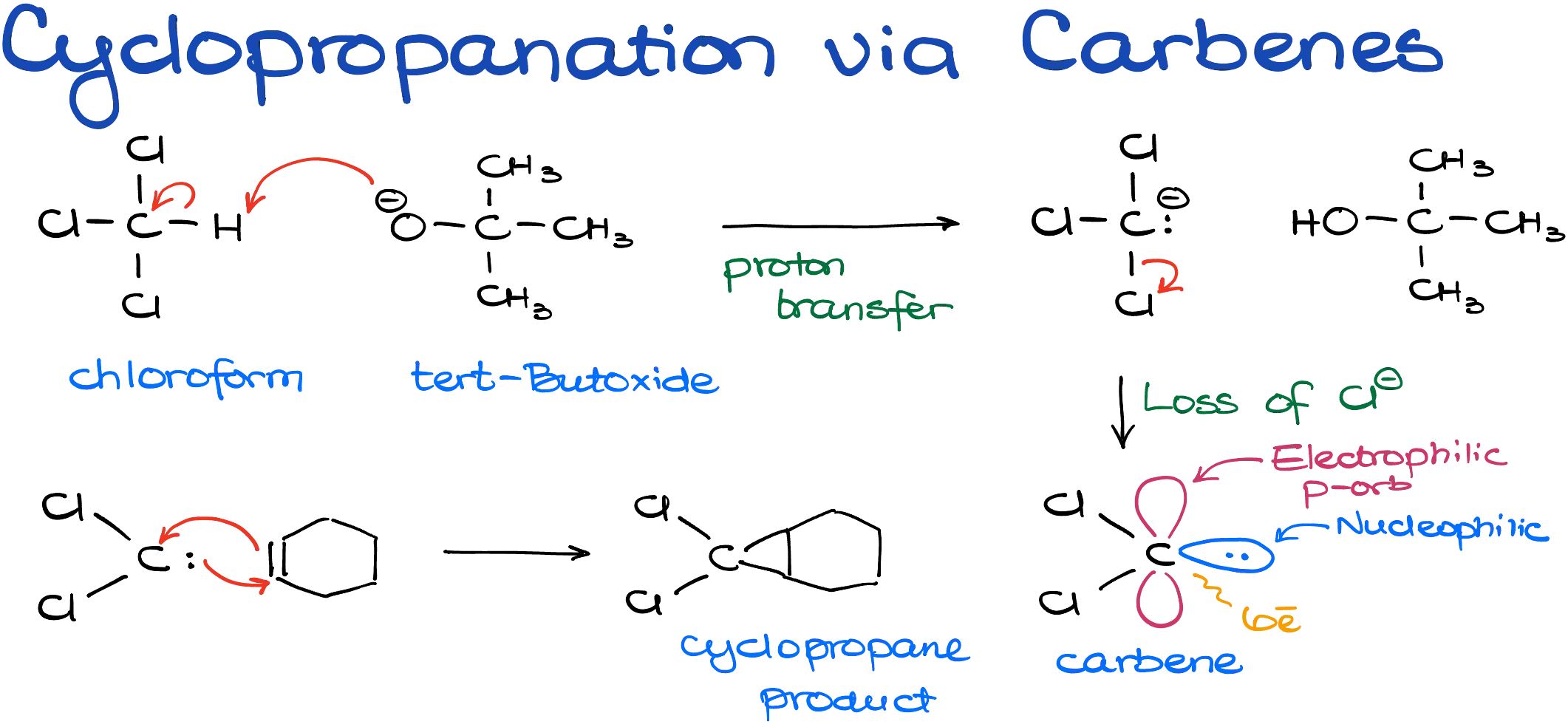
There are various ways to create carbenes, but in a typical sophomore organic chemistry course, we usually focus on the generation of carbenes from chloroform (CHCl₃) using a strong base like sodium or potassium tert-butoxide. In the first step, the base abstracts a proton from chloroform, forming a negatively charged intermediate. This intermediate quickly eliminates a chloride ion, forming the neutral carbene.
Carbenes are unique and somewhat exotic species in organic chemistry. They are neutral six-electron carbon atoms that behave both as nucleophiles and electrophiles. They have a lone pair of electrons, making them capable of donating electrons, and they also have an empty p-orbital, making them excellent electron acceptors. This dual personality allows them to react with alkenes by both giving and taking electrons, forming cyclopropane rings in a single concerted step.
Examples of Cyclopropanation
These reactions often produce substituted cyclopropanes with two geminal chlorine atoms, a feature that can be quite useful for further chemical transformations.
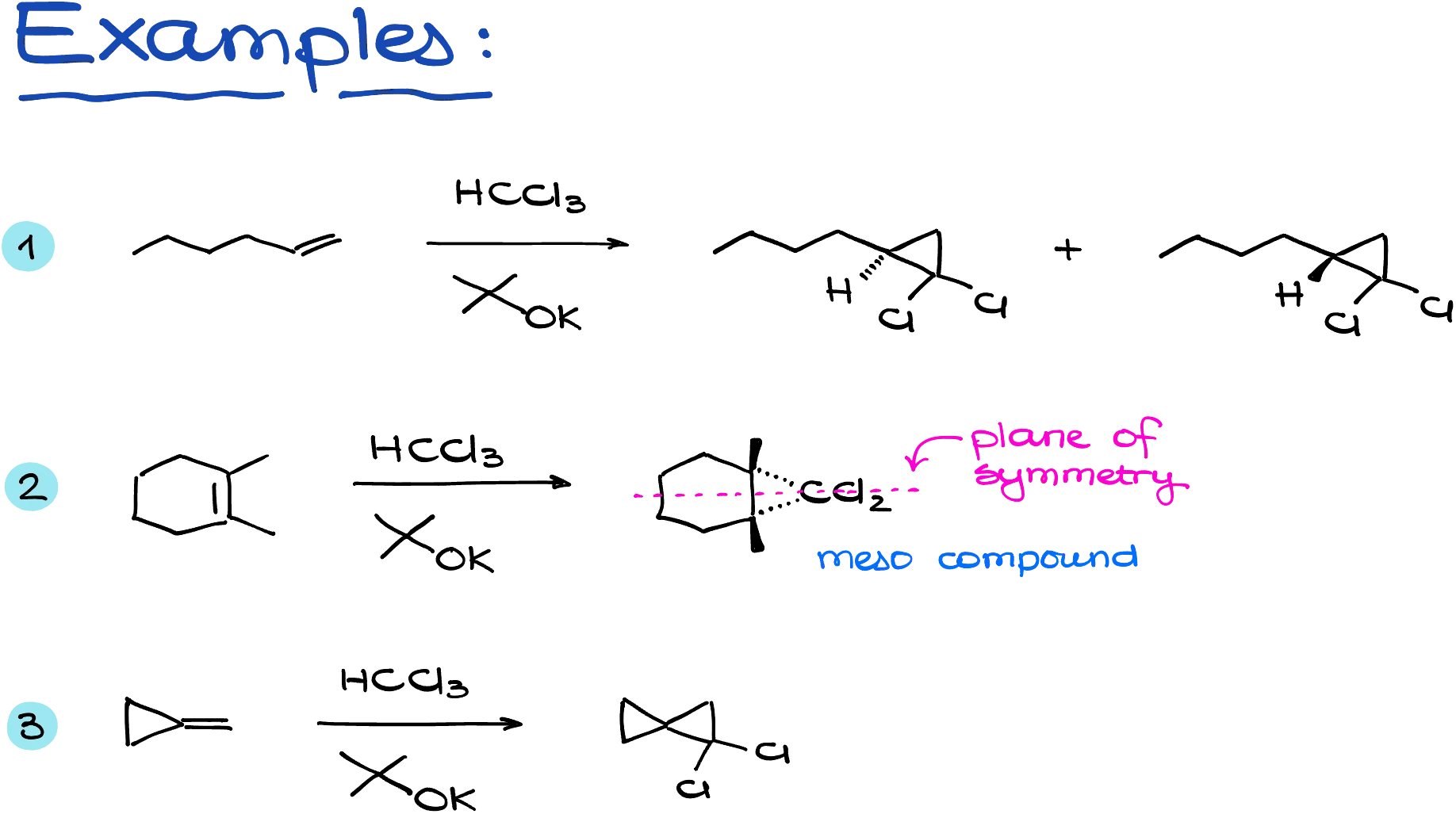
In one example, adding a carbene across a double bond creates a chiral cyclopropane, giving two enantiomeric products. We show this by indicating the configuration of the implicit hydrogen: one with the hydrogen pointing toward us (on a wedge), and the other with it pointing away (on a dash). In another example, the product turns out to be a meso compound due to an internal plane of symmetry, so we don’t expect any stereoisomers. And in the final case, we form a spirocyclic molecule, which is also not chiral and therefore also lacks stereoisomers.
Recap
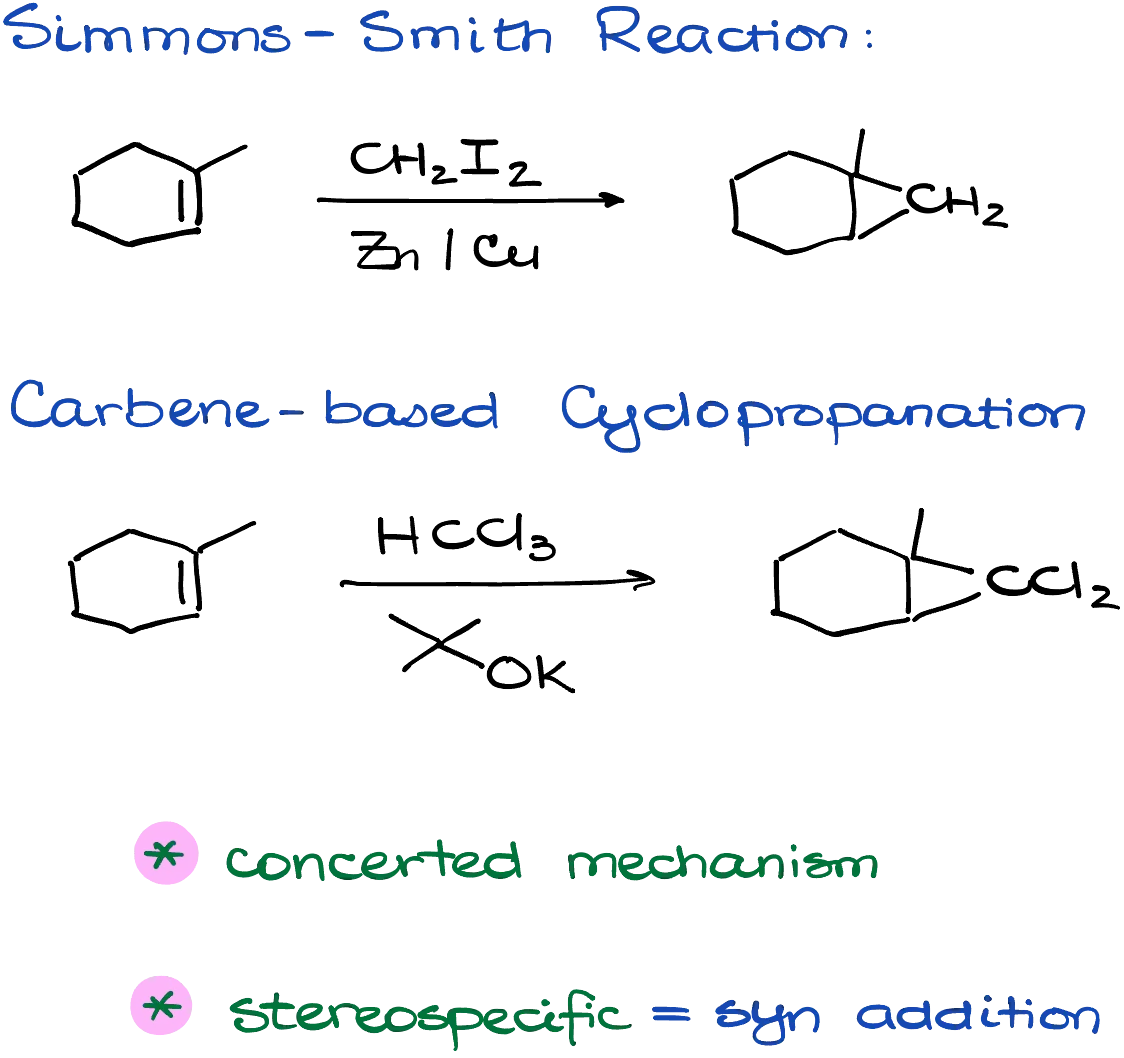
To sum it all up: the Simmons–Smith reaction uses zinc and diiodomethane to produce cyclopropane rings from alkenes through a zinc-containing carbenoid intermediate. The “regular” cyclopropanation reaction involves the formation of a true carbene, often from chloroform and tert-butoxide, and results in a cyclopropane with two geminal chlorine atoms. Both reactions proceed via concerted mechanisms and are stereospecific, yielding syn addition products.
Practice Questions
Answers
Would you like to see the answers and check your work?
Sign up or login if you’re already a member and unlock all members-only content!