Hydrohalogenation of Dienes (1,2- vs 1,4-Addition)
Conjugated alkenes have a lot of similarities with regular alkenes. However, due to the conjugated nature of the molecule, they have specific reactivity that we do not see in isolated alkenes. One of the typical reactions of alkenes—hydrohalogenation—is also something that we see for the dienes as well. However, you no longer can approach it as a simple “Markovnikov’s addition” reaction. Hydrohalogenation of dienes brings its own set of challenges. So, let’s talk about the details of this reaction and why it is different from a simple hydrohalogenation.
Hydrohalogenation of Butadiene
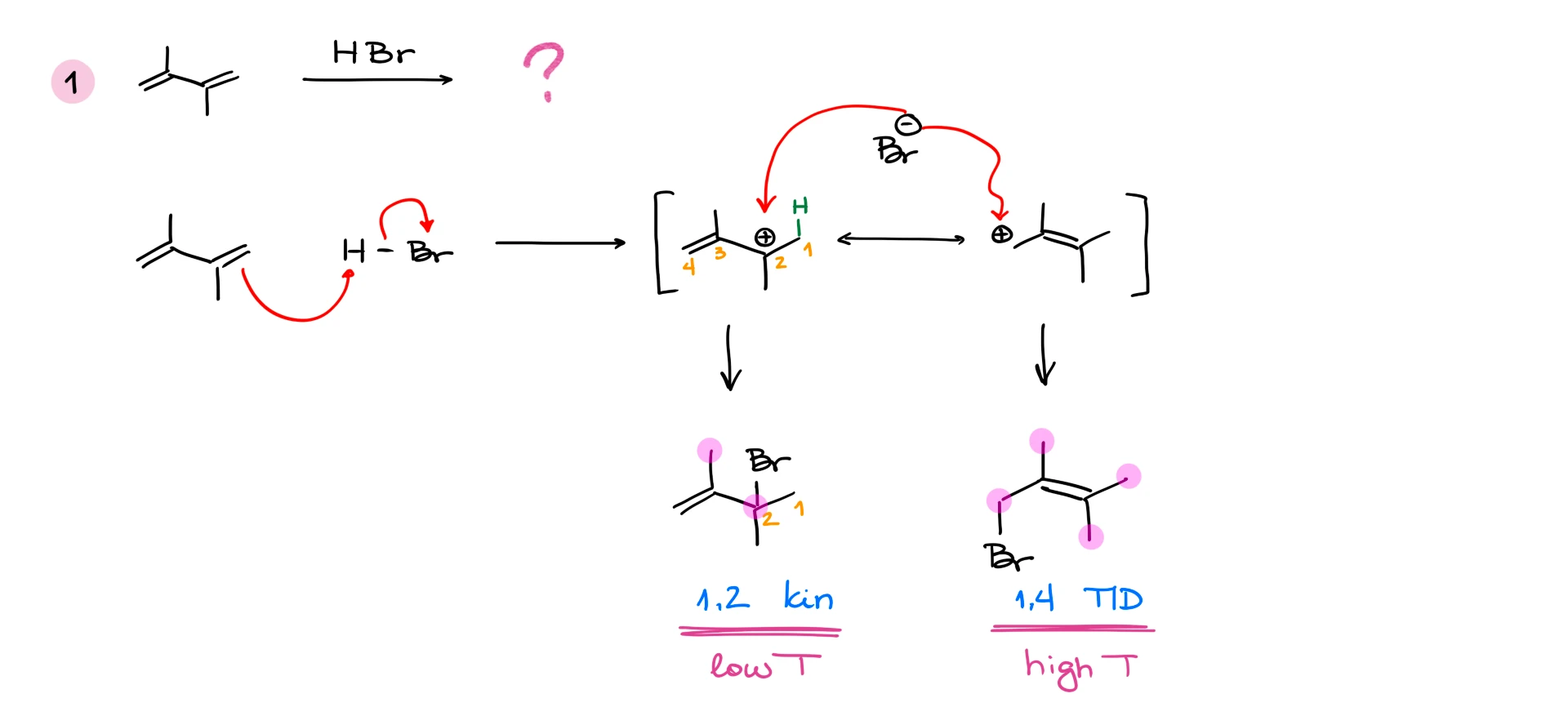
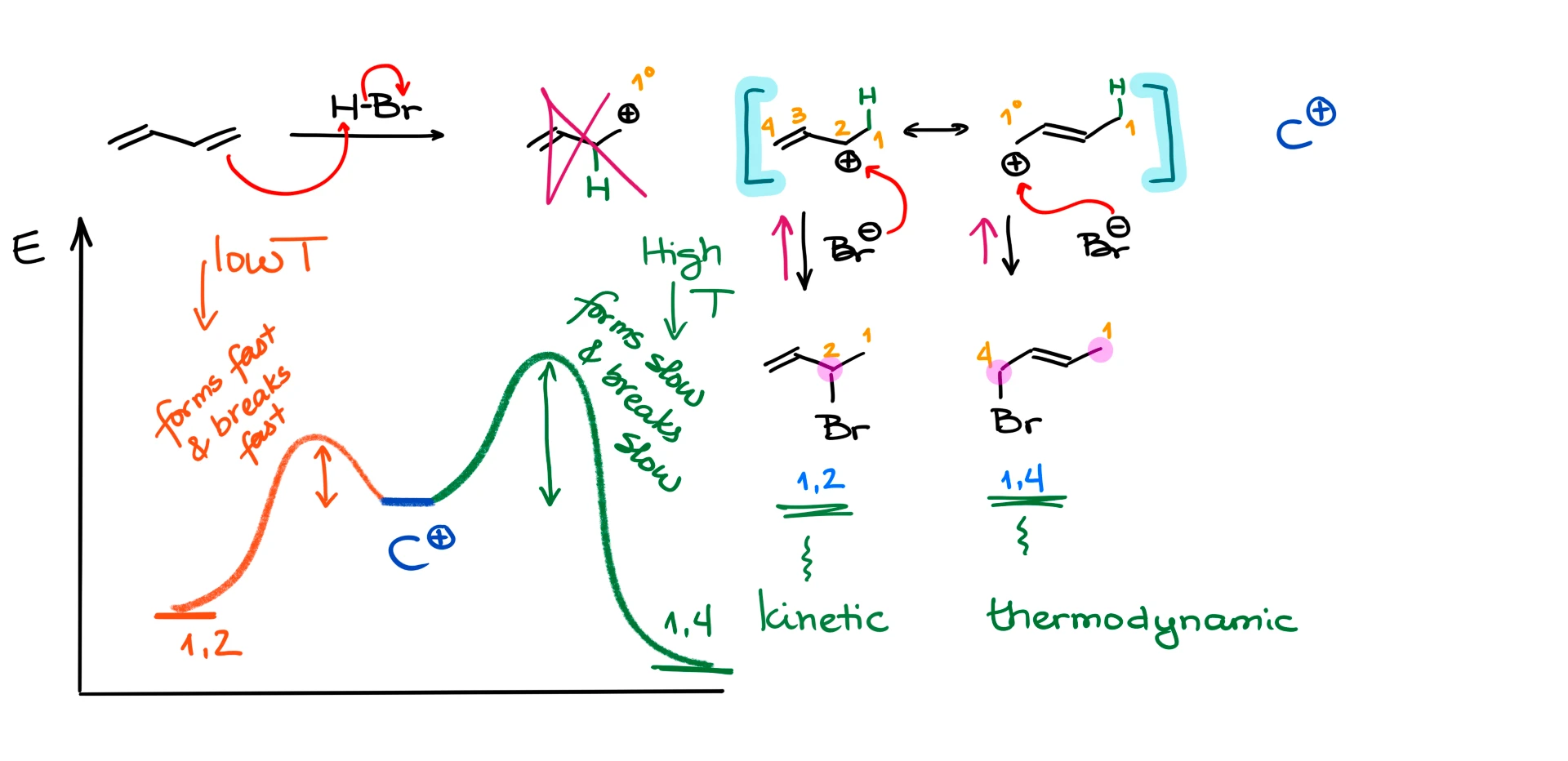
Let’s first look at the hydrohalogenation of butadiene. If we react it with hydrogen bromide (HBr), we’ll start with the electrophilic attack on the diene. In this case we can make two different carbocation intermediates. One—a primary (1°) carbocation, and another one—a secondary (2°) allylic carbocation.
Naturally, the primary carbocation is very unstable, so we would not expect that to be our actual intermediate. The secondary carbocation in this example is much more stable. But one more thing that we want to keep in mind here is that this is an allylic carbocation. This means that it is stabilized by resonance. So, we can represent this carbocation with two resonance contributors. I also want to point out that due to the resonance stabilization, you should always opt for the allylic carbocation. This is going to be true even if you’re comparing the allylic carbocation with a tertiary (3°) carbocation that is not allylic.
Now, since we have two possible places where the nucleophile can attack, we’re going to end up with two different products. One will be a result of the bromide attack at the secondary position, and the other one—attack at the primary carbon. You know what’s even more interesting? These two constitutional isomers are not going to be formed in the same quantities either. But before we talk about the major and the minor product in this reaction, let’s quickly discuss how we’re going to refer to them.
1,2- vs 1,4-Product
The carbon where we attach our hydrogen is going to be our carbon #1. This numbering system has nothing to do with the IUPAC nomenclature. It’s just the way we’re going to number the molecule for the purposes of this reaction. If we continue numbering through the rest of the molecule, then we’ll see that the bromine atom will always end up on either carbon #2, or carbon #4. So, we’re going to refer to these products as a 1,2-product, and 1,4-product, solely based on the location of the hydrogen and the bromine atoms.
Kinetic vs Thermodynamic Products
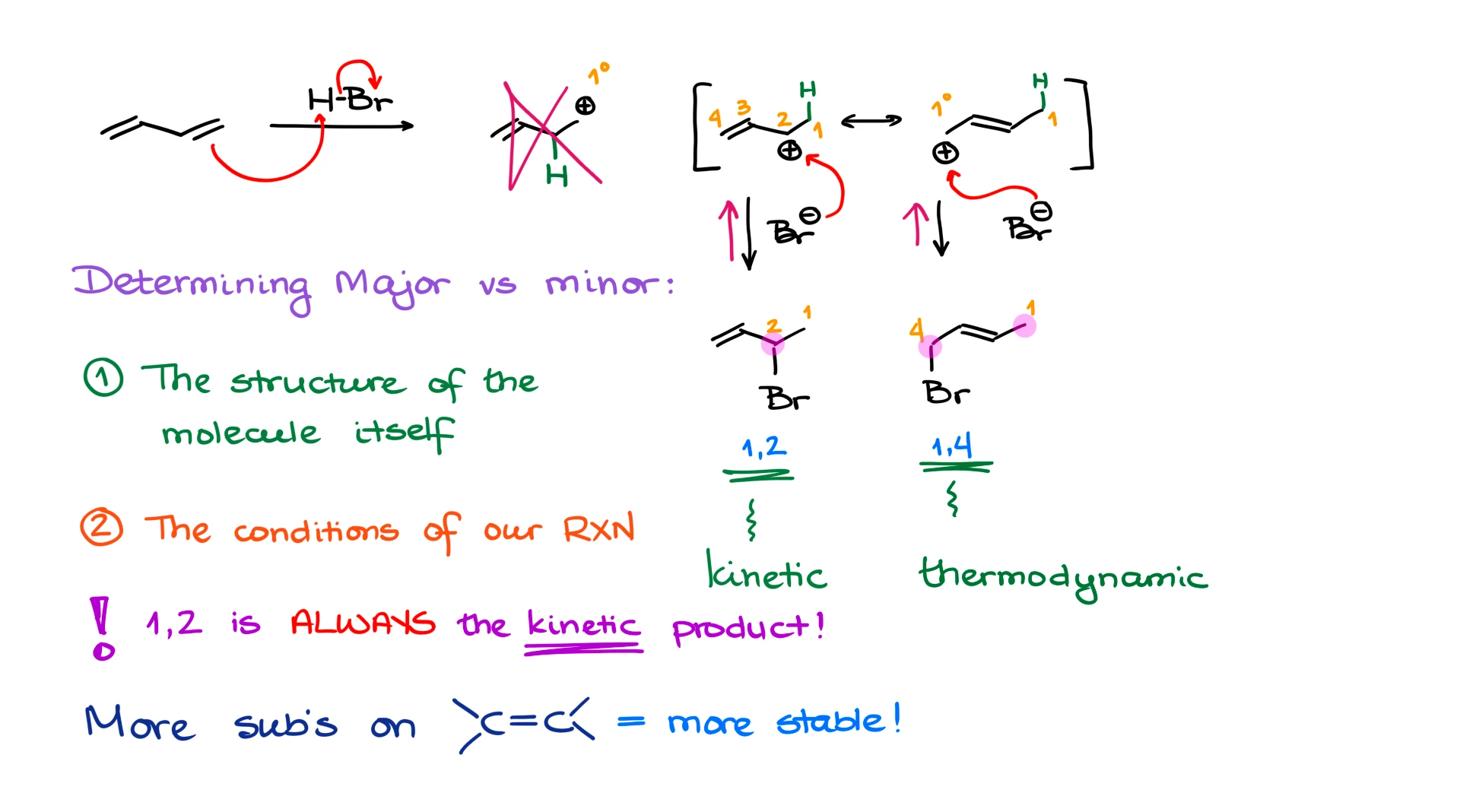
Now, returning back to our discussion of the major vs minor products, it’s unfortunately more complicated than just pointing at one of them. The major vs minor product in this reaction will depend on two factors:
- The structure of the molecule itself, and
- The conditions in which we perform this reaction.
Let me elaborate on this. Let’s get back to our mechanism. The 1,2-product forms very fast. And therefore, we call it a kinetic product. Why does it form so fast? Well, the HBr molecule exists as a “tight ionic pair” meaning that the bromide ion is always nearby the proton. And once the electrophilic attack makes the corresponding carbocation, the bromide anion is right next to it and therefore will quickly react with the carbocation.
So, remember, in a hydrohalogenation of dienes, the 1,2-product forms faster and is called the kinetic product. This is always going to be true.
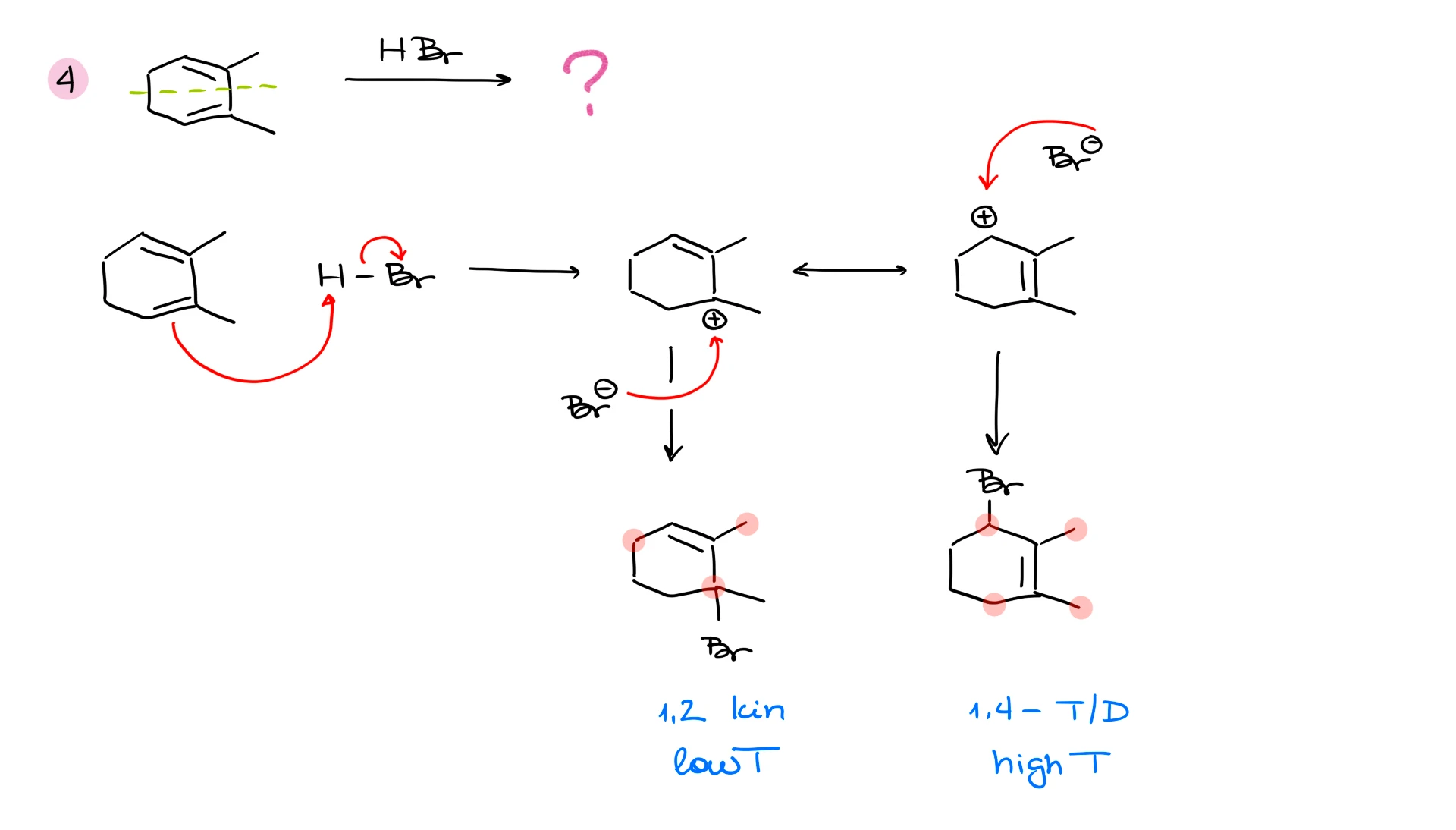
What about the other product? The 1,4-product in this particular example, is going to be called a thermodynamic product because it is more thermodynamically stable. And how do I know that? To assess the thermodynamic stability of products in this type of reaction, we need to look at the double bond. We know that the more substituents we have on the double bond, the more stable it’s going to be (in most cases). So, in the case of the 1,4-product in this reaction, the double bond has two substituents on it, while the 1,2-product only has one substituent on the double bond.
Well, why does it matter? We know that the 1,2-product forms faster, so who cares about the 1,4-product, right? Not quite. You see, this reaction is an equilibrium. And since we have a resonance-stabilized carbocation as an intermediate, the products can easily reform this intermediate. Which means, that even though the 1,2-product forms faster than the 1,4-product, it can just as easily break up and make the other isomer.
Let’s analyze this situation from the kinetics and thermodynamics perspective. If we have our carbocation intermediate, it can quickly make 1,2-product, or it can make the 1,4-product but slower. However, because the thermodynamic product is lower in energy and is more stable, it will also dissociate slower. So, in other words, if we make a thermodynamic product, it will be less likely to break up than the kinetic product. While the kinetic product forms fast and breaks fast as well. This means that with time, we’re going to be accumulating the thermodynamic product and losing the kinetic product.
So, if we stop reaction quickly or do it at a lower temperature, we’re going to predominantly get the kinetic product. But if we give our reaction time to reach equilibrium or do it at a higher temperature, we’ll get predominantly the thermodynamic product.
Does this mean that the 1,4-product will always be the thermodynamic product? ABSOLUTELY NOT! This is one of the most common misconceptions in these reactions! The more stable product is the thermodynamic product. And it depends on the diene structure.
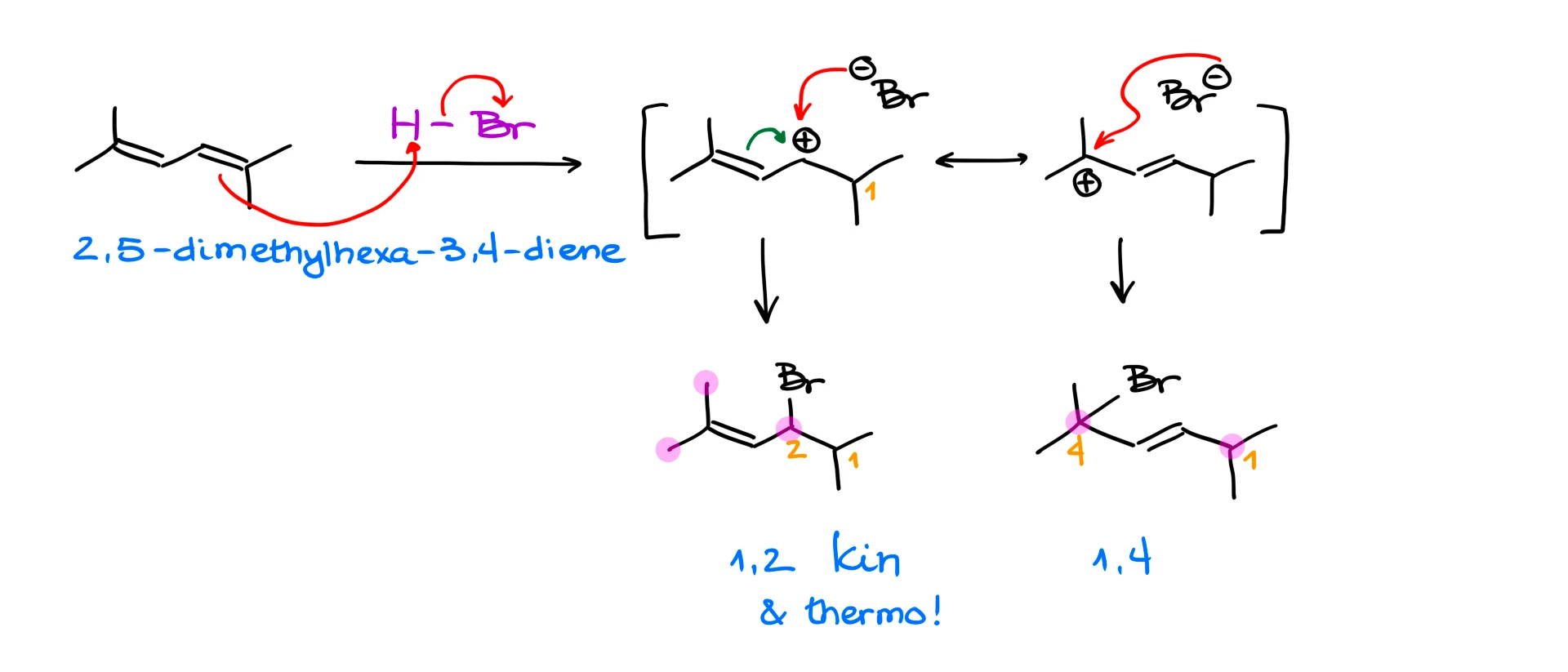
For instance, if I react 2,5-dimethylhexa-3,4-diene with HBr, we’ll get 1,2-product, which is our kinetic product, and we’ll get 1,4-product. But if we look at the double bond in this case, we’ll see that the double bond in the 1,2-product has three substituents, while the double bond in the 1,4-product only has two. This means that the 1,2-product in this case is both kinetic and thermodynamic! This means that 1,2-product here will always be the major product regardless of the conditions. So, always make sure to analyze your molecules and never blindly assign the kinetic and thermodynamic products before you look carefully at your molecules!
Practice Questions