Molecular Representations
Organic chemistry, as a science, has its own “written language” that we use to communicate the ideas in OChem. You have already seen molecules before in general chemistry and are familiar with some types of molecular representations. Let’s go over the typical ways we draw molecules in organic chemistry and discuss their pros and cons.
Molecular Formulas
Molecular formulas show us the number and ratio of atoms in a molecule. So, by looking at the molecular formula you can easily determine how many atoms of each type you have in the molecule’s composition.
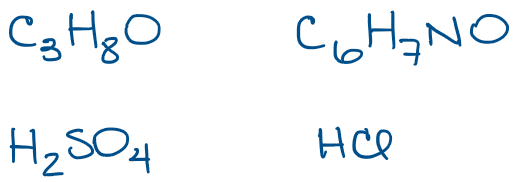
Some small molecules, like HCl or H2SO4 are simple enough, so we know that those are the hydrogen chloride (or hydrochloric acid when in solution) and sulfuric acid. Those can’t really represent anything else, so it’s easy to guess what the molecule is. THe other two examples, however, are much trickier. Take, for instance, the first molecule I have in this set: C3H8O, this seemingly simple molecular formula can actually represent multiple different molecules. In fact, C3H8O can be one of 3 completely different molecules with different names, structures, physical and chemical properties!
Molecular formulas are, however, the least useful type of molecular representation in organic chemistry. Organic chemistry is all about structure. So, by not showing the molecular structure, you can’t actually tell what the molecule is and what properties it may have.
Lewis Structures
There are two types of Lewis structures that we use for the molecular representations.
Complete Lewis Structures
There are full (complete) and condensed Lewis structures. While the full structures have their uses, they are rarely used for molecular representations in organic chemistry. We do however use condensed structures from time to time.
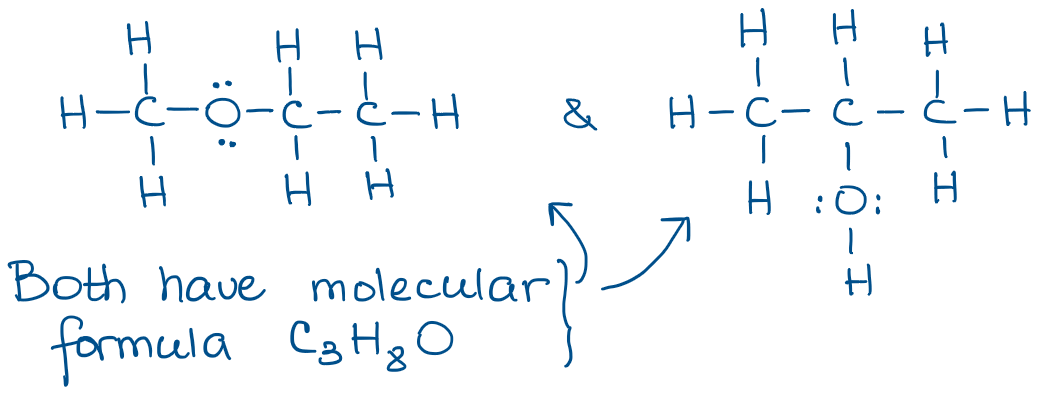
The most valuable aspect of the complete Lewis structures as they show all bonds and non-bonding (spare) electrons in the molecule. The big issue with the Lewis structures is that they take a very long time to draw. It’s ok for one or two molecule, but when you have to draw a few dozens, it becomes very tedious. And, as you can imagine, you’ll be drawing a bunch of molecules in organic chemistry! Here’s an example:
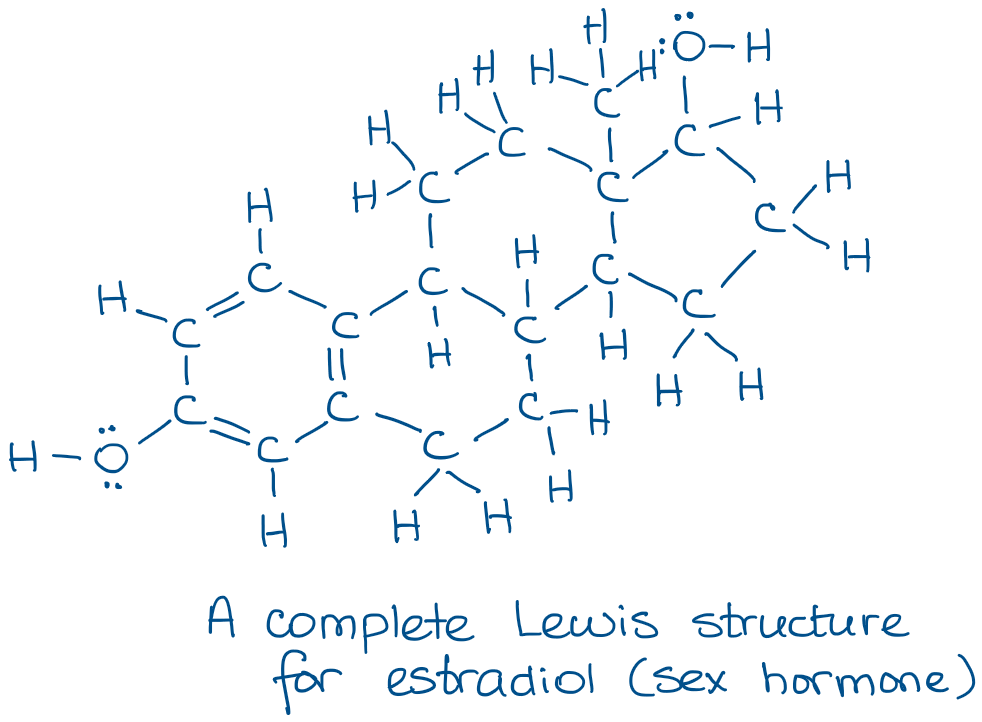
Try copying the molecule above a few times and see how much time it’ll take you. 😹 It’s also easy to lose some atoms especially in big molecules. And lost atoms will lose you points on the test. 😭
Condensed Lewis Structures
Condensed structures, however, are more common in organic chemistry as they are much easier and faster to draw. Here are a couple of example of the molecules from above:
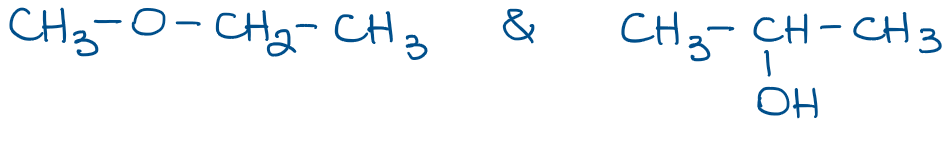
In condensed Lewis structures we only show the bonds between the groups of atoms connected to each other. In the example above, the first molecule has a methyl group (CH3) shown as a group. It’s the implicit assumption that hydrogens are attached to carbon, which in turn is connected to the rest of the molecule through the bond that we show. We also often skip the implicit electron pairs on heteroatoms like O or N. We’ll only show those when they are important for a reaction or a mechanism we’re discussing.
We also tend to skip the bonds between the groups of atoms in long chains. For instance, look at the molecule of 2-methylhexane, which can be shown with or without bonds between the groups.
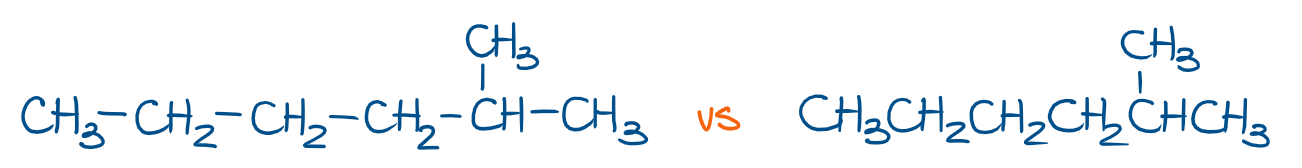
In the left example, you show all bonds between the groups. In the right example, we don’t show the bonds, so you gotta keep those in mind when thinking about your molecule.
Skeletal (Bond-Line) Structures
Skeletal structures is the most common type of the molecular representation in organic chemistry. In the skeletal structures we show the absolute minimum that we need to represent a molecule in a non-ambiguous way. Thus, skeletal drawings have certain rules we use to draw them:
- Carbons and Hydrogens are implicit. We dont show hydrogens attached to carbons and we only show bonds connecting carbons as lines.
- If you have heteroatoms like O or N, you must show them and you must show hydrogent on those atoms too (if any).
- Electron pairs are optional (often implicit), however, charges are not. So, it’s ok not to show the electrons but you absolutely must show the charges like a ⊕ or a ⊖ on the corresponding atoms in your molecule.
Thus, the Lewis structures that we’ve seen before:
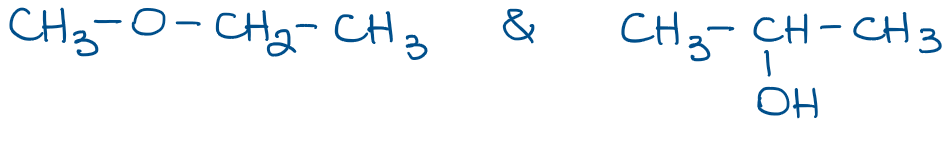
Can be drawn using the skeletal structures as following:
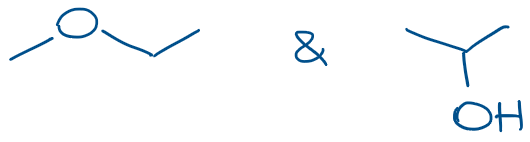
Skeletal structures are much faster and easier to draw, so those are the preferred method of molecular representation in organic chemistry. You definitely wanna make sure you know how to interpret those if you wanna ace your tests!
Do’s and Don’ts of the Lewis Structures
While we may not be showing all bonds in the condensed Lewis structures, it’s still important to imply the proper connections in the molecule. For instance, the ethyl group (CH3CH2—) connects to the parent chain through the second carbon and not the first one, or somewhere in the middle. So, it’s important to orient it properly in space when you’re showing it connected to the parent.
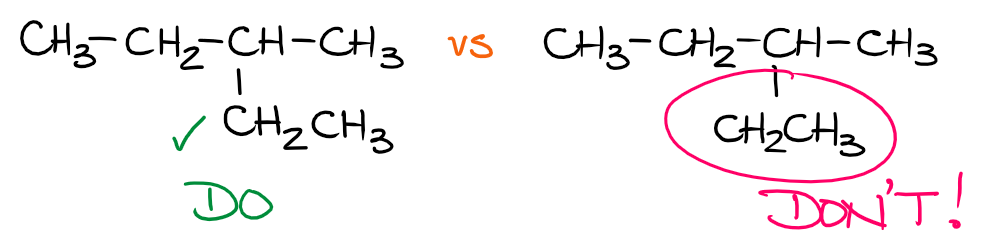
The first molecule (the left one) shows the proper connection through the carbon atom which is actually connected directly to the parent. The second molecule, however, has the ethyl group connected to the parent somewhere in the middle of the group. This is a big no-no and many, if not most, instructors will punish you for that. This is a very silly mistake that is very easy to avoid. So, make sure you pay attention to it.
In my experience, students who make improper connections in condensed Lewis structures struggle with understanding what the structure actually means. So, if that’s you, make sure you step back and review the whole concept of Lewis structures, what they mean, and what information we get from those.
Another example of this type of a mistake is when the group is connected to the parent through some sort of heteroatom. For instance, in ethers, you have an oxygen atom playing the role of this chain link connecting to carbon-containing parts in the molecule.
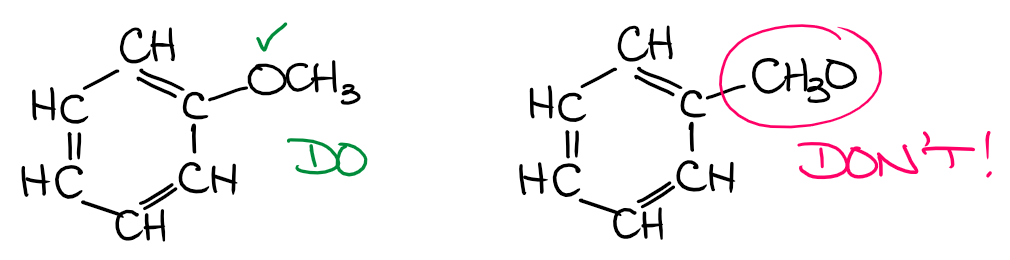
In this case, while we may refer to the ether appendix as the CH3O or methoxide, we still need to make sure we show it connected to the rest of the molecule via O and not C! Knowing your functional groups will definitely help you here. 😉
Connections of Functional Groups
Most functional groups have an atom which is going to be the one connecting the functional group to the rest of the molecule. This way, if you know your functional groups, you’ll always know which atom to use to connect that group to the parent chain. Let’s look at the nitro group as an example:
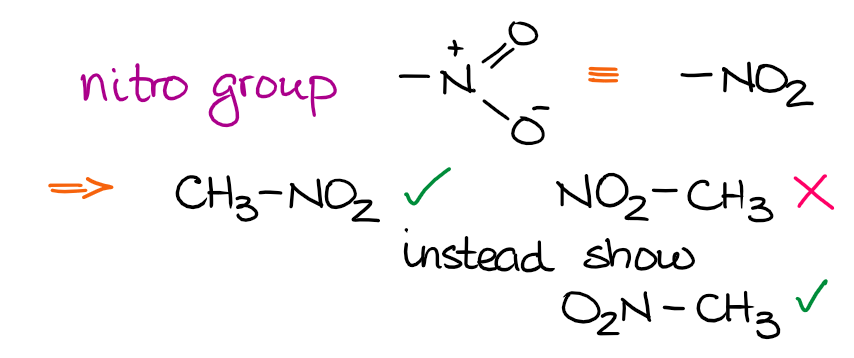
The nitro group uses the nitrogen atom as the anchoring point to connect to the rest of the molecule. Thus, it’s incorrect to draw it connecting through the O’s. For smaller functional groups such as nitro group, your instructor might easily overlook it. However, if you take some other classic functional groups like, say, a carboxylic acid, then drawing it incorrectly will definitely cost you some points!
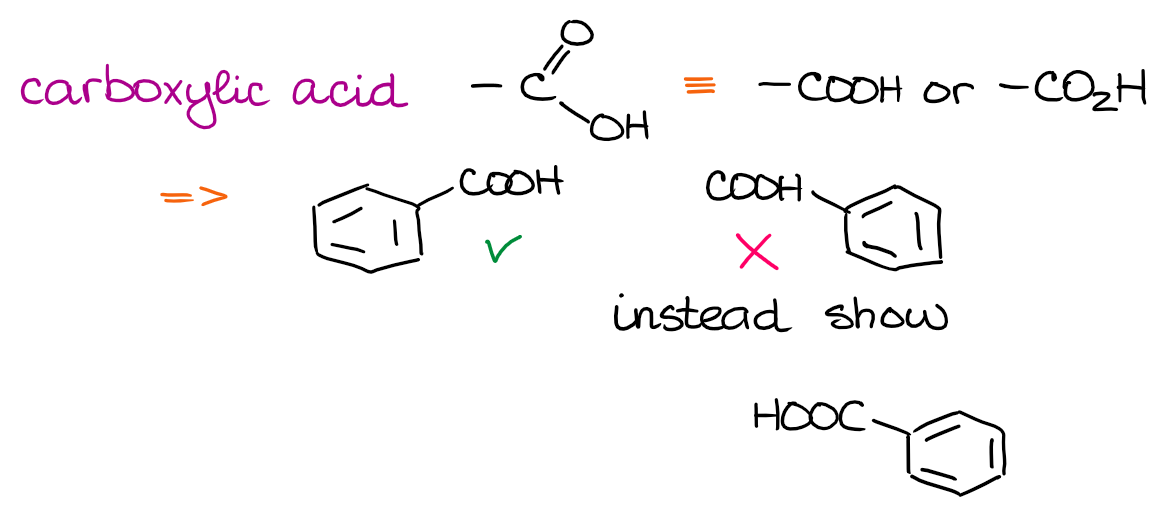
When I was an instructor, I mercilessly gave zeros for the COOH “attached” through hydrogen. You might think it’s not that big of a deal and everyone understands what you mean. And you would be correct. As an instructor, for instance, I can understand exactly what you mean by those improper drawings. But then again, this is the written language of organic chemistry. You won’t expect your English teacher to overlook you spelling things incorrectly in your paper, nor should you expect your chemistry teacher overlook chemistry “spelling” mistakes.
Do’s and Don’ts of the Skeletal Structures
When drawing the skeletal structures, remember, that each dot connecting the lines is a carbon atom. For instance, if we wanna draw a line representation of 2-butanol, we’ll get the following picture:
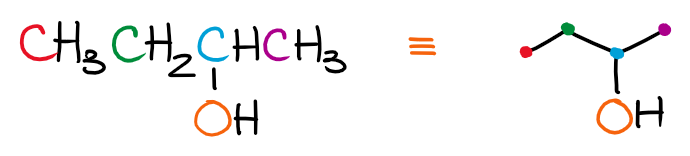
Each carbon is a dot. I’ve highlighted those with different colors to make it a little easier to track. Notice how we do show the oxygen as that one is the heteroatom and thus is no longer implicit. We also show the hydrogens on that oxygen for the same reason. Only hydrogens on carbons are implicit and don’t need to be shown.
As you’re drawing the structures using the mere lines, it’s a good idea to keep a few rules of thumb in mind while at it. First of all, try to adhere to realistic bond angles. Remember that the sp3 species have an angle of 109.5°, sp2 has a bond angle of 120°, and sp is linear with the bond angle of 180°. Thus, we draw zig zags for the single and double bond and straight angles for the triple bond:
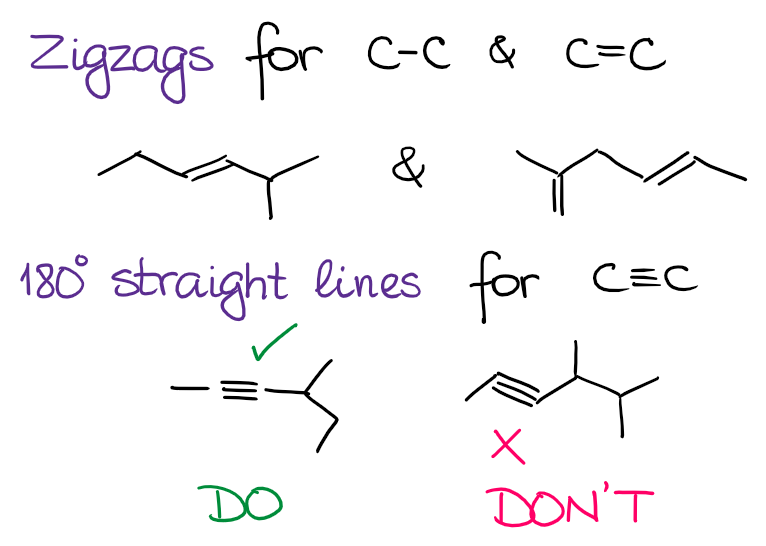
While it’s not strictly speaking incorrect to show a triple bond on the zig zag, it’s a little of a pet peeve for many instructors. And while you probably won’t losing any points drawing your structures like that, it will annoy your instructor.
I do recommend you stick to 180° angles for sp-hybridized atoms as this would give you a better representation of the structure. It’ll also give you a better idea of how some reactions may proceed if you have a proper angles in the molecule.
So, we do the zig zags for the main chain. Good. Now, how do we deal with the substituent groups hanging off the main chain?
Proper Way of Showing Substituents on the Chain
Generally, you want to show them away from the angle made by the carbon atom of the zig zag-ing parent chain:
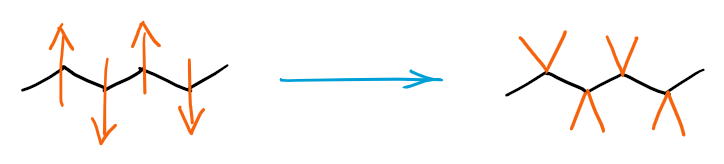
Making a habit of drawing the substituents on the same side away from the angle will also make it easier for you to draw proper stereochemical structures later in the course.
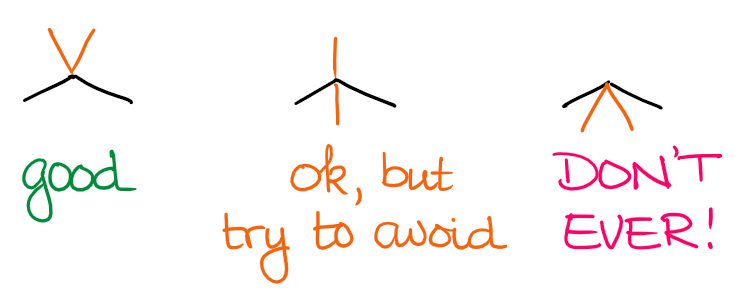
Again, while this is not a huge dealbreaker, it’s an annoyance for most instructors. I rarely see students lose points over those mistakes, but it does happen. It also usually is the sign that you may not have a firm enough grasp on the relation of the 2D drawing and 3D structure of the actual molecule.
